This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Researcher helps boost immune system memory against influenza
When humans or animals get infected, the body's immune system tries to not only clear the infection but also build up a memory of the pathogen that caused it. So, when the pathogen comes around again for possible reinfection, the body has an army of memory T cells that can recognize and destroy it. These T cells are a critical part of immunological memory, and an important component of efficient vaccines.
Now, researchers at the University of Missouri are one step closer to making the T cell army stronger. In a recent study, conducted in the Roy Blunt NextGen Precision Health building, the researchers found that by manipulating one molecular signaling pathway in the T cells that participate in clearing influenza virus in the lungs, the strength and longevity of immunological memory produced can be improved.
This finding can potentially support the future development of more effective vaccines and therapeutics to combat influenza and other respiratory infections with the ultimate goal of increasing the body's immunological memory, which can both prevent and lessen the severity of infections and reinfections.
Emma Teixeiro and Mark A. Daniels, associate professors in the MU School of Medicine, led the study, which involved unique mouse models of influenza infection.
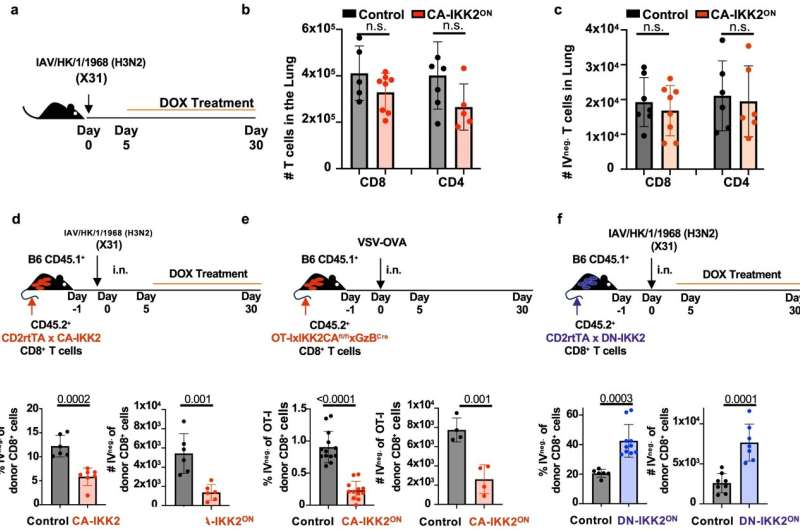
"Immunologists like myself have always wondered why T cells in the lungs after influenza infection disappear so quickly," Teixeiro said. "This research can help us solve that problem by increasing the amount of T cells that can fight against infection. In this study, we have identified novel ways to improve the generation and long-term maintenance of protective immunity against influenza, and that is by manipulating a molecular target known as the IKK2/NFkB signaling pathway."
Teixeiro added that T cells can recognize parts of viruses that do not mutate, so if researchers can better understand how to strengthen the T cells and extend the timeframe when they can do their job appropriately, the body's immune system will ultimately be better suited to fight against infection and lessen the severity.
While the influenza virus was the focus of this particular study, gaining knowledge of the underlying molecular mechanisms and signaling pathways that regulate memory in tissues can be helpful to improve therapeutics for patients with cancer, autoimmunity or other respiratory infections.
"By unveiling the biochemical and molecular secrets of these T cells, we can provide valuable information to other scientists who work on optimizing vaccine strategies," Teixeiro said. "The good news is there are already clinical treatments that do target this particular pathway we identified, so this study is a big step in the right direction, but we still have a long way to go."
"IKK2/NFkB signaling controls lung resident CD8+ T cell memory during influenza infection" was recently published in Nature Communications. Co-authors on the study include Curtis J. Pritzl, Dezzarae Luera, Karin M. Knudson, Michael J. Quaney, Michael J. Calcutt and Mark A. Daniels.
More information: Curtis J. Pritzl et al, IKK2/NFkB signaling controls lung resident CD8+ T cell memory during influenza infection, Nature Communications (2023). DOI: 10.1038/s41467-023-40107-1