This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Combination radiation with immunotherapy shows promise against 'cold' breast cancer tumors
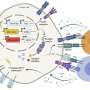
Researchers at Weill Cornell Medicine have discovered that radiation therapy combined with two types of immunotherapy—one that boosts T cells, and another that boosts dendritic cells—can control tumors in preclinical models of triple negative breast cancer, a cancer type that's typically resistant to immunotherapy alone. Immunotherapy activates the body's own immune system to fight cancer but isn't effective for difficult-to-treat "cold" tumors, like this.
The findings were published Aug. 24 in Nature Communications. Though radiation therapy has previously been combined with T-cell boosting immunotherapy, it rarely succeeds in eliminating cold tumors. The new, preclinical study found that activating another type of immune cell called a dendritic cell, in addition to the other two approaches, produced a synergistic effect that elicited tumor regression.
"I think this is quite exciting," said principal investigator Dr. Sandra Demaria, professor of radiation oncology at Weill Cornell Medicine and pathologist at NewYork-Presbyterian/Weill Cornell Medical Center, who conducted the research under the auspices of the Department of Radiation Oncology. "There is so much room for improvement to provide more effective therapeutic options, especially for patients with cold tumors."
Infiltrating cold tumors
Cold tumors are often referred to as impregnable fortresses that can't be infiltrated by the immune system's T cells, which directly attack viruses, bacteria, as well as cancer cells. That makes them tough to treat with an immunotherapy drug called checkpoint inhibitors. Checkpoints are a safety mechanism on T cells that stop the immune cells from attacking healthy cells, but some cancers use them to hide from the immune system. Taking the brakes off these checkpoints with inhibitors allows the immune system to find and destroy cancer cells.
The team's previous research explored using radiation therapy to "warm up" cold tumors. Radiation directly kills cancer cells and sends out inflammatory signals that attract "killer" T cells. While this approach activated the immune system, the T cells couldn't overcome the immune suppression of the tumor. They also knew from previous studies that an inhibitor targeting the CTLA4 checkpoint could be combined with radiation to trigger a stronger immune response.
For the new study, the researchers used two preclinical models of triple-negative breast cancer, which is aggressive and difficult to treat. These tumors are resistant to immunotherapy and poorly infiltrated by T cells. While the combination of radiation with CTLA4 inhibition converted cold tumors into T-cell-inflamed tumors, this was not enough to significantly reduce the tumor. They also found that adding a second checkpoint inhibiting immunotherapy did not improve tumor responses. Next, Dr. Demaria and her colleagues decided to look beyond T cells.
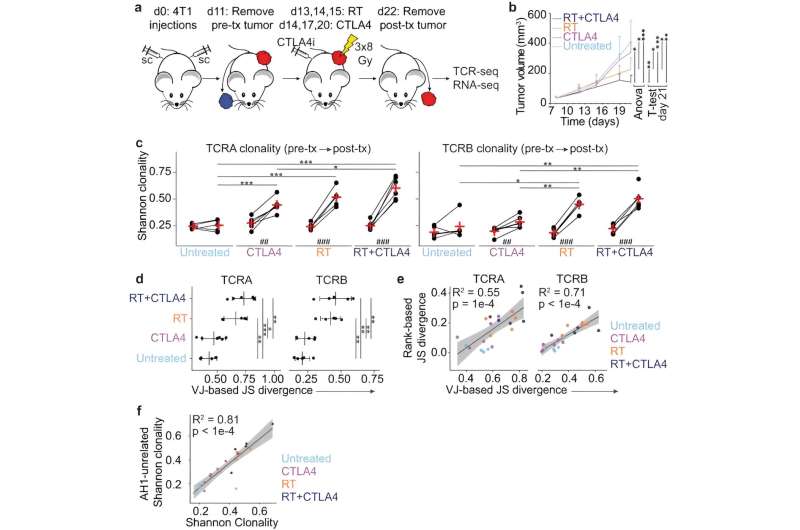
Finding help for T cells
The researchers then explored ways to boost dendritic cells, part of the immune system's first line of defense against cancer and infections. Dendritic cells can engulf and digest cancer cells, and then present the pieces (antigens) to an army of T cells. This activates T cells to specifically target the tumor.
To do this, they added CD40 agonist, an antibody that stimulates dendritic cells, in addition to the combination of radiation treatment and CTLA4 inhibitor. "We saw a dramatic difference. The majority of tumors went away," said Dr. Demaria, who is also a professor of pathology and laboratory medicine at Weill Cornell Medicine.
The three-pronged therapy resulted in complete or almost complete elimination of targeted tumors, as well as partial control of tumors that were not targeted by the radiation. However, she cautioned that the approach was not effective in eliminating micrometastases that had spread to the lungs, even though it showed increased immune activation in the area. Dr. Demaria will continue studying how the combined therapy interacts within the lungs and other organs.
She said the study is a good reminder to look beyond T cells in immunotherapy. "I think tumor immunologists are a little bit T-cell-centric. T cells have many brakes but releasing multiple brakes does not help when the engine is not running properly. We must start thinking more carefully about what's missing and consider the partners for T cells," said Dr. Demaria, who is also a member of the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine.
Dr. Silvia Formenti, chair of the department of radiation oncology and associate director for translational research at the Meyer Cancer Center, Weill Cornell Medicine, and the breast cancer research team are planning to integrate these promising findings in a clinical trial to test the combination of radiation therapy, anti-CTLA4 and CD40 agonist in metastatic breast cancer patients.
More information: Nils-Petter Rudqvist et al, Immunotherapy targeting different immune compartments in combination with radiation therapy induces regression of resistant tumors, Nature Communications (2023). DOI: 10.1038/s41467-023-40844-3