This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
New methodology to study the intricacies of amyloid plaques in Alzheimer's disease
Researchers have now combined STED microscopy, a technology that allows superresolution visualization, and a recently created new antibody to observe the amyloidogenic aggregates characteristic of Alzheimer's disease. The work, led by scientists from the Universitat Autònoma de Barcelona (UAB), the Karolinska Institute (KI), and the biotechnology company BioArctic, both in Sweden, has surpassed the capabilities of conventional confocal microscopy, and will allow further study of the structure and morphology of amyloid deposits and the mechanisms involved in their formation.
In the brains of patients with Alzheimer's disease, there are accumulations of plaques of beta-amyloid (Aβ) protein aggregates, which have been linked to tissue deterioration and brain malfunction. The major component of these plaques are chains of about 40–42 amino acids, which with conventional confocal light microscopy can only be described in collective terms as amorphous, dense, or diffuse plaques.
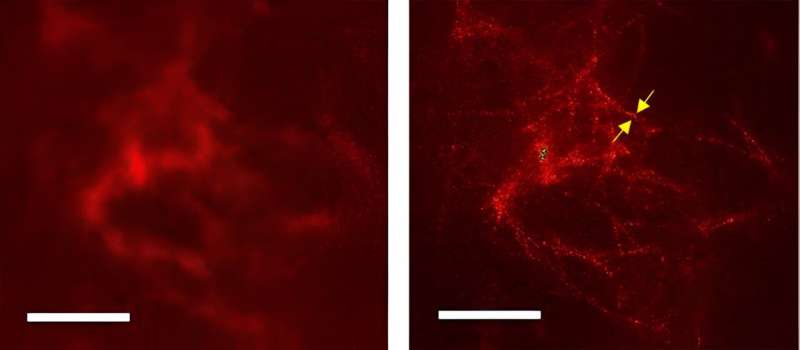
This classical view is far from the individual fibers seen with electron microscopy. With electron microscopy, they appear threadlike, between 6 and 10 nanometers in diameter, unbranched and often formed by filaments coiled around each other. However, although electron microscopy offers higher resolution, it also has several drawbacks, including a very high cost and a sample preparation process that increases the risk of artifacts.
In the study, the research team has evaluated the use of a third type of microscopy—STED (stimulated emission depletion)—to examine the structure and morphology of Aβ aggregates. Using this technology, developed by Nobel laureate SW Hell, the scientists examined brain sections from Alzheimer's disease model mice, along with a new recombinant human antibody labeled with fluorescence that selectively reacts with Aβ aggregates.
Published in the journal Cell & Bioscience, the work describes details of plaque structure that had not been resolved with conventional light microscopy. "We have achieved a spatial resolution that exceeds 5 to 10 times the capabilities of conventional confocal light microscopy, both in in vitro samples and in brain tissue sections, and we have been able to discern individual fibers within plaques, a milestone previously only possible with electron microscopy," explains Dr. Björn Johansson, first author of the paper and researcher at the KI Department of Clinical Neuroscience.
"This is an important advance in the field, which will allow us to further characterize the mechanisms involved in Aβ deposition in plaques and its subsequent removal," adds Vladana Vukojevic, co-author and researcher at the same department.
Lydia Giménez-Llort, co-author of the study and researcher at the Department of Psychiatry and Legal Medicine and the Institute of Neuroscience of the UAB, says, "The ability to obtain these images in animals that had been under behavioral observation will allow us to better understand the development and progression of cognitive and neuropsychiatric symptoms, with the aim of finding significant biochemical and neuropathological correlates."
The authors agree that previous studies of Alzheimer's disease conducted with conventional light microscopy lack information which can now be addressed with this new methodology, on the role of beta-amyloid aggregates in the pathogenesis of the disease. "STED microscopy is emerging as an indispensable tool to drive scientific progress in Alzheimer's research," they conclude.
More information: Björn Johansson et al, The interwoven fibril-like structure of amyloid-beta plaques in mouse brain tissue visualized using super-resolution STED microscopy, Cell & Bioscience (2023). DOI: 10.1186/s13578-023-01086-4