This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Applications of macrocyclic molecules in cancer therapy: Target cancer development or overcome drug resistance
A study, by Professor Xiaoling Song, Professor Biao Jiang and Pr. Xiaoling Song and Ph.D. student, Yifan Wu (Shanghai Institute for Advanced Immunochemical Studies, ShanghaiTech University), on the application of macrocyclic molecules in cancer treatment has been published in MedComm—Oncology.
Macrocyclic compounds are cyclic molecules with a structure of 12 or more atoms. In the past decades, macrocycles have received increasing attention in drug development. The U.S. Food and Drug Administration has approved several macrocyclic drugs for cancer therapy. However, the importance of this class of cancer drugs is still not widely known.
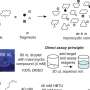
Song and Wu comprehensively summarized the applications of both FDA-approved macrocyclic drugs in the past 15 years and those promising macrocyclic small molecules currently under development for cancer therapy, providing strong evidence for the applications of macrocyclic compounds in cancer treatment.
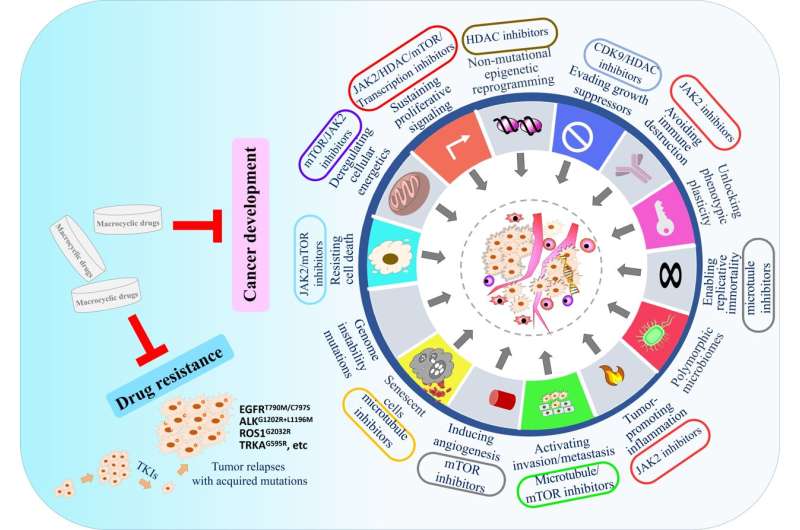
Firstly, macrocyclic molecules can target proteins critical for tumorigenesis or cancer development. Macrocyclic compounds can block nearly 80% of cancer's signature characteristic events to control cancer occurrence and progression, suggesting a space for using macrocyclic compounds in cancer therapy.
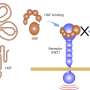
Secondly, macrocyclic compounds can effectively overcome drug resistance caused by on-target mutations in targeted cancer therapy.
Small compact macrocyclic molecules can enter the binding pocket, bind to mutant proteins, and inhibit their activities, especially those with resistant mutations around the binding pocket. These macrocyclic compounds developed recently for overcoming cancer drug resistance include the pyrazole- or pyrimidine-based TRK inhibitor Selitrectinib and the next-generation ROS1/ NTRK inhibitor Repotrectinib (TPX-0005).
Additionally, the authors review the ongoing clinical trials for these novel macrocyclic drug candidates, and some of them have shown excellent therapeutic efficacy. For example, recent data from the latest Phase 1/2 clinical trial showed that first-line use of repotrectinib in ROS1-positive TKI-naïve lung cancer patients resulted in long-term progression-free survival (PFS) of 35.7 months (TRIDENT-1, NCT03093116.
Finally, Song and Wu provided novel insights into macrocyclic molecular drugs regarding their future directions, limitations, and combinational therapy with other drugs in clinics. This comprehensive review is beneficial for the development of novel cancer treatment strategies.
More information: Xiaoling Song et al, Applications of macrocyclic molecules in cancer therapy: Target cancer development or overcome drug resistance, MedComm—Oncology (2023). DOI: 10.1002/mog2.50