This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Side-effect avoiding treatment shows early promise against breast cancer in mice
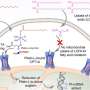
New experimental evidence suggests that substances known as narrow-spectrum Wnt signaling inhibitors—which could have fewer side effects than other related substances—are capable of suppressing the growth of breast cancer tumors in mice. Aina He of Shanghai Jiaotong University Affiliated Sixth People's Hospital, China, and colleagues presented these findings November 9 in the open access journal PLOS Biology.
While certain subtypes of breast cancer can be targeted with special medications, others can only be treated with standard chemotherapy. For some patients, chemotherapy may lead to the growth of stem cell-like cancer cells that are drug resistant.
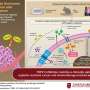
Previous studies suggest that medications that inhibit a specific biological process called Wnt signaling could potentially combat these cells, but so far, the potential benefits of Wnt signaling inhibitors have been hampered by their damaging side effects, particularly on bone density.
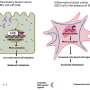
These side effects arise from the fact that humans have ten different versions of the Wnt signaling receptor, Frizzled, with distinct functions. Researchers have therefore recently developed new Wnt signaling inhibitors that could reduce side effects by targeting just three of these receptors. However, it has been unclear how effective these narrow-spectrum Wnt signaling inhibitors might be at treating cancer.
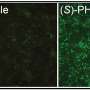
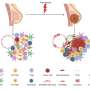
To shed new light on the subject, He and colleagues conducted a series of experiments with a specific narrow-spectrum Wnt signaling inhibitor known as TcdBFBD, which was derived from a toxin found naturally in the bacterial species Clostridium difficile. They tested TcdBFBD in several different mouse models that mimic different types of breast cancer—basal-like and luminal-like—found in humans.
The researchers found evidence suggesting that TcdBFBD suppressed tumor growth and reduced the activity of stem cell-like cancer cells in the mice, without side effects on bone density. They also found evidence that TcdBFBD can synergize with the standard chemotherapy drug cisplatin to inhibit both basal-like and luminal-like breast cancer tumors in mice.
These findings provide preliminary evidence for the potential therapeutic promise of narrow-spectrum Wnt signaling inhibitors like TcdBFBD. However, more research will be needed to investigate their effectiveness in humans, examine how they might synergize with other cancer treatments beyond cisplatin, and explore their effects in additional types of cancer—such as serous ovarian cancer and oral squamous cell carcinoma.
The authors add, "A bacterial toxin fragment targets and suppresses breast cancer tumor-initiating and chemo-resistant cells."
More information: Targeted inhibition of Wnt signaling with a Clostridioides difficile toxin B fragment suppresses breast cancer tumor growth. PLoS Biology (2023). DOI: 10.1371/journal.pbio.3002353. journals.plos.org/plosbiology/ … journal.pbio.3002353