This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Biomedical engineers unveil the dynamics of maternal immune responses
Sepideh Dolatshahi, an assistant professor of biomedical engineering at the University of Virginia, is spearheading an exploration of systems immunology in its crucial development phase—during pregnancy.
Systems immunology is about unraveling concealed patterns within the human immune system, said Dolatshahi, whose approaches to her research span computational modeling, systems serology and cutting-edge spatial analysis techniques to investigate immune interactions between mother and fetus during pregnancy that could later support early childhood immunity.
Designing tailored and effective vaccine plans
Babies are immunocompromised and rely on antibodies from their mothers to protection against infections. Mothers are vaccinated during pregnancy to help increase and customize the antibodies passed on to the baby. While early vaccines such as tetanus, diphtheria and pertussis (TDAP), and recently respiratory syncytial virus (RSV), have shown astounding success, the number of vaccines available to mothers is limited and babies remain vulnerable to many pathogens.
The Dolatshahi lab is working to shed light on how vaccines given to pregnant women interact with both their changing immune system and the developing placenta to confer immune protection in the baby. The team's goal is to design personalized vaccination strategies that can be especially beneficial to vulnerable patients, for example, those who have genetic complications or compromised immune systems.
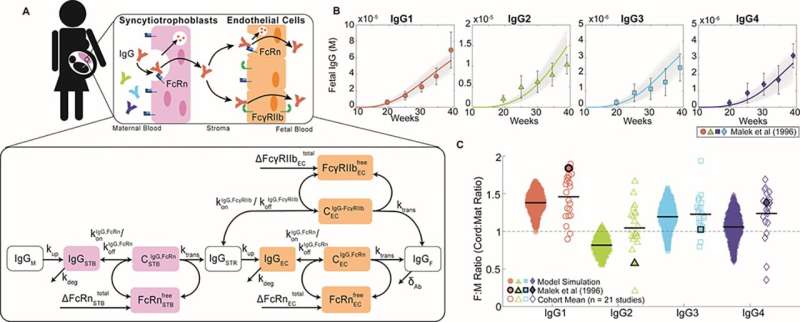
To accomplish this, Dolatshahi and her colleagues developed the first computer-based predictive model of maternal vaccination and placental antibody transfer. This model now serves as a tool to help doctors and pre-clinical researchers test different vaccination strategies in silico before embarking on costly, tedious clinical trials, and to shorten the timeline for when patients might benefit from the resulting immunization protocols.
The importance of placental development
The placenta is a protective gatekeeper that safeguards the fetus, governing which substances can reach the growing baby. Shockingly, little is known about the specifics of how the placenta regulates the passage of antibodies from mother to baby.
To shed light on this process and understand which interactions are responsible for conferring transfer of protective antibodies to the baby, Dolatshahi's team is taking a closer look at the important molecules expressed by placental cells and how they work together to transport antibodies.
By examining this specific process using spatial analysis tools, they hope to uncover information that will identify new targets of maternal vaccines that will maximize the benefit to the baby.
Uncovering variability across the population
During pregnancy the mother's immune system is in constant flux, so there is no reason to believe that vaccination during pregnancy should be a one-size-fits-all approach. Dolatshahi and her team hope to uncover key variables affecting placental antibody transfer that might contribute to differences across the population.
By defining what causes some babies to receive more maternal antibodies than others, Dolatshahi's work will help doctors identify patients who may benefit from specially designed vaccines to best support their baby's immune system. More broadly, this investigation will shed light on the biological and socioeconomic factors which make some women more prone to immune-related pregnancy complications.
This research stands as a milestone to understanding the nuances of how maternal antibodies traverse the placenta and confer protection in newborn babies, and to uncovering specific immune-related mechanisms of pregnancy complications such as preterm birth and preeclampsia. Dolatshahi's research sets the stage for a more profound comprehension of the immune system's behavior during pregnancy, offering a promising trajectory for further advancements in this critical domain.
The team's publication about this research, "Quantitative mechanistic model reveals key determinants of placental IgG transfer and informs prenatal immunization strategies," has been accepted for publication by PLOS Computational Biology.
More information: Remziye E. Wessel et al, Quantitative mechanistic model reveals key determinants of placental IgG transfer and informs prenatal immunization strategies, PLOS Computational Biology (2023). DOI: 10.1371/journal.pcbi.1011109