This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Review highlights the challenges and recent advances in targeted therapies for lupus nephritis
Patients with lupus nephritis (LN), a severe complication of systemic lupus erythematosus (SLE), often undergo progressive kidney damage, with approximately 20% of these patients advancing to end-stage renal disease.
The current therapeutic landscape for LN, dominated by glucocorticoids and immunosuppressants, is limited by suboptimal response rates, the risk of disease flare-ups, and adverse effects, accentuating the necessity for safer and more effective treatment modalities.
In the latest issue of the Chinese Medical Journal, a review authored by Dr. Wei Chen from the Department of Nephrology of the First Affiliated Hospital, Sun Yat-Sen University, provides a comprehensive overview of the targeted therapies for LN in clinical settings.
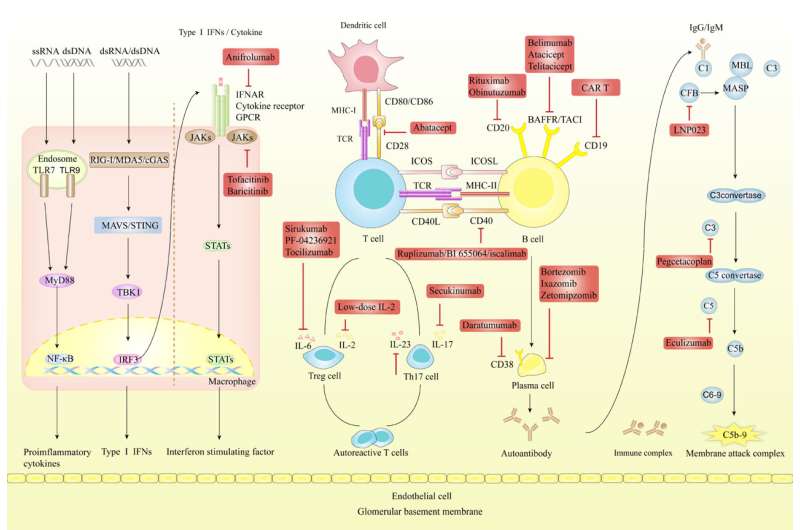
LN presents a multifaceted pathogenesis characterized by an accumulation of immune complexes and a malfunctioning complement system. The early stages of LN are defined by the infiltration of innate immune cells like neutrophils, macrophages, and dendritic cells. These cells incite local inflammation and tissue damage via the release of pro-inflammatory mediators. Progression of the disease involves B-cell dysregulation, leading to improper production of autoantibodies.
A critical aspect of LN's progression is the dysregulated activation of self-reactive T-cells, facilitated by antigen recognition through the T-cell receptor and augmented by co-stimulation through CD28 and B7 interactions. This suggests the potential for therapeutic strategies that concurrently target multiple aspects of LN's underlying mechanisms.
Therapeutic strategies for LN increasingly focus on B-cell targeting, employing monoclonal antibodies (mAbs) techniques to deplete B-cells or suppress B-cell activity. Rituximab, an anti-CD20 mAb, has been effective in markedly reducing B-cell counts and enhancing renal function.
Consequently, it is recommended as a supplementary therapy for persistent or recurrent LN in key guidelines, including the Kidney Disease Improving Global Outcomes (KDIGO) 2021 and the European League Against Rheumatism/European Renal Association–European Dialysis and Transplant Association (EULAR/ERA-EDTA) 2019.
Other therapies such as Belimumab targeting BAFF to suppress B-cell survival, are effective in improving SLE outcomes and renal responses, making it the sole FDA-approved targeted therapy for LN. Atacicept and Telitacicept targeting BAFF and APRIL show promise but require further validation through LN-specific clinical trials.
Additionally, therapies like Daratumumab effectively target plasma cells, and proteasome inhibitors such as Bortezomib are emerging as potential treatments for autoimmune diseases, including LN.
Dr. Chen says, "Chimeric antigen receptor-modified T (CAR-T) cells, engineered to target CD19 and other B-cell surface antigens, have emerged as promising alternatives to T-cell depletion. Preliminary evidence from animal models and case reports suggests that CD19-directed CAR-T cells outperform anti-CD19 antibodies in depleting CD19-positive B-cells, both in terms of efficacy and duration."
Dysfunctional T-cell activation, particularly through the CD40- CD40L interaction, is a key factor in the pathogenesis of LN. Clinical trials investigating a first-generation anti-CD40L antibody, Ruplizumab, aimed at this interaction were halted due to severe thromboembolic complications. Simultaneously, trials for second-generation anti-CD40 antibodies, such as BI 655064, intended to reduce thromboembolic risks, have not shown significant efficacy in treating LN.
Patients with LN often present with elevated levels of interferons (IFNs), pro-inflammatory interleukins (ILs), and tumor necrosis factors, which stimulate the JAK/STAT signaling pathway. This upregulation suggests these molecules as potential therapeutic targets.
Nonetheless, several JAK inhibitors, including Deucravacitinib, Olcitinib, R333, and Filgotinib, have either been ineffective or discontinued due to adverse side effects. Additionally, IL-6 targeted therapies, such as Sirukumab, PF-04236921, and Tocilizumab, have not provided substantial clinical trial evidence regarding their safety or efficacy in LN treatment.
"Given the complex and heterogeneous nature of LN, multiple factors may account for trial failures," Dr. Chen says, emphasizing the importance of rigorous patient selection, including stratification through genetic and biomarker analyses, for evaluating targeted therapies. "Other key elements include the integration of investigational agents into established standard-of-care protocols, vigilant surveillance for adverse events, and consideration of comorbidities."
Overall, a summary of the latest updates in targeted therapies in LN may imply that clinical trial failures underscore gaps in our understanding of LN pathogenesis. As genomic sequencing and high-throughput technologies advance, new therapeutic targets and mechanisms may emerge, providing further insight into the disease and its treatment.
More information: Xiuzhi Jia et al, Targeted therapies for lupus nephritis: Current perspectives and future directions, Chinese Medical Journal (2023). DOI: 10.1097/CM9.0000000000002959