This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
HOXA9 tracking reveals RBM5 dual function and therapeutic potential for acute myeloid leukemia
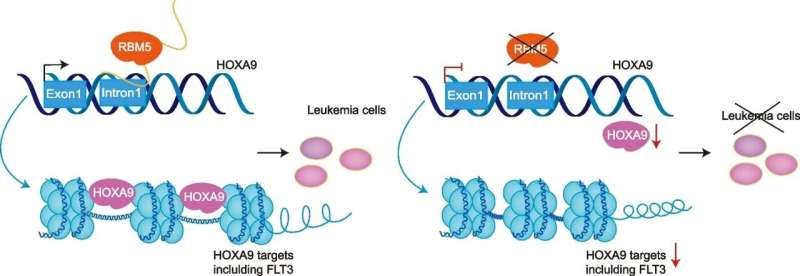
The protein HOXA9 is overexpressed in most acute myeloid leukemia (AML) cases and is associated with poor patient outcomes. However, HOXA9 is a difficult protein to target therapeutically, so researchers at St. Jude Children's Research Hospital looked for ways to extinguish it indirectly.
Using CRISPR/Cas9 screening, the researchers identified RBM5, demonstrating a causative link between RBM5 expression and leukemia cell proliferation. This link is driven by a novel dual function of RBM5 as both a DNA and RNA handler in gene expression. The research was published today in Genome Biology.
Overexpression of the protein HOXA9 is a hallmark of AML, present in over 70% of cases, often with poor prognosis. While this would implicate it as a useful drug target, the protein's role as a transcription factor has left it "undruggable" because a drug that interferes with HOXA9 would likely have numerous other off-target effects.
This inspired researchers to approach the problem differently by investigating the proteins HOXA9 works alongside and relies on to function. Chunliang Li, Ph.D., St. Jude Department of Tumor Cell Biology, co-corresponding author on this paper, is one such researcher. Through his recent work devising an unbiased CRISPR screening strategy to identify targets of HOXA9, he uncovered a network of opportunities.
"This has been a continued effort since my lab was established in 2017," said Li, "We built up this unique reporter system in early 2019, which is the first reporter authentically representing HOXA9 expression in these leukemia systems."
The CRISPR/Cas9 screening approach is elegantly simple in design but incredibly effective. It involves attaching a fluorescent tag to the HOXA9 gene and inserting it into leukemia cell lines. This enables researchers to track differences in expression levels by looking at fluorescence in cells.
"We wanted to identify a more targetable or novel regulator. So, we conducted an unbiased whole genome CRISPR screening to target all the genes expressed in cells," Li stated. This allowed the researchers to examine different pathways where HOXA9 left its fluorescent fingerprint.
To the researchers' surprise, splicing factors appeared to be the most represented pathway.
"This was quite surprising to us because splicing factors regulate different combinations of the transcript, but not usually the level. Our data suggested these proteins control the HOXA9 expression level," said Li. "So, we hypothesized maybe the splicing factors have another function, like a dual function."
The protein that stood out was the RNA-binding protein RBM5. The researchers found that RBM5 is highly expressed in leukemia cells as opposed to other cell types and that both the DNA- and RNA-binding sites are vital to its oncogenic functions. While the RBM family comprises vital RNA splicing factors, their function in DNA transcription was unknown. To address the direct transcriptional regulation of RBM5/HOXA9, the researchers generated a system to allow the acute degradation of RBM5.
"Immediately after RBM5 protein was removed from cells, HOXA9 mRNA levels were significantly reduced," Li explained, "This reduction happened as early as two hours later but did not impact splicing events of HOXA9." Additionally, leukemia cells stripped of their ability to produce RBM5 were rescued through overexpression of HOXA9, further demonstrating the link between the two proteins.
These results have Li looking to explore the protein as a drug target to treat AML.
"We think RBM5 is a very good dependency gene, which should be a good target based on our functional assays," he said. "If we can specifically target the DNA binding affinity of these proteins, we should be able to combine with other existing therapies in synergy to target HOXA9-driven leukemia."
More information: Mengli Zhang et al, RNA-binding protein RBM5 plays an essential role in acute myeloid leukemia by activating the oncogenic protein HOXA9, Genome Biology (2024). DOI: 10.1186/s13059-023-03149-8