This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Research discovers disrupted cellular function behind type 2 diabetes in obesity
Disrupted function of "cleaning cells" in the body may help to explain why some people with obesity develop type 2 diabetes, while others do not. A study from the University of Gothenburg describes this newly discovered mechanism.
It is well known that obesity increases the risk of insulin resistance and type 2 diabetes. It is also well known that some people who gain weight suffer from the disease and others do not. The reasons for these differences are not clear, but they are related to the function of the adipose tissue rather than the amount of body fat.
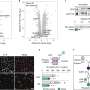
The study, published in the journal PNAS, is mainly based on experiments in mice, but the research indicates that the newly discovered mechanism also applies to humans.
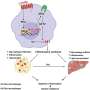
Weight gain increases the breakdown of the structural protein collagen to make room for the growing fat cells within adipose tissue. Collagen is a natural building block in the body that provides strength to cartilage, muscles, and skin.
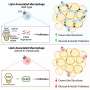
The breakdown of collagen is handled by macrophages, a type of white blood cell that is part of the immune system. Macrophages are involved in the destruction of invading bacteria, but they also engulf and digest damaged cells and debris such as degraded collagen in adipose tissue during weight gain.
The macrophage function is impaired in obesity
The collagen is fragmented by enzymatic degradation outside the fat cells, and the collagen fragments are then engulfed by macrophages for complete degradation. What this study shows and describes is how highly regulated this uptake of collagen fragments is.
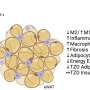
And it is fast when it works properly. However, this function of macrophages was found to be deactivated in obesity and insulin resistance, leading to the accumulation of collagen fragments in adipose tissue.
While this has not been considered a problem until now, the study shows that collagen fragments are not just debris, but actively influence various cellular processes such as inflammation and cell division.
The process thus goes from maintaining normal adipose tissue function during weight gain, to becoming pathogenic in some cases. When samples of human macrophages were exposed to diabetes-like conditions, they also lost their ability to "clean up" collagen.
Identification and prevention
The study was carried out by a research team at the Sahlgrenska Academy, University of Gothenburg, led by Ingrid Wernstedt Asterholm, Professor in Physiology.
"When adipose tissue grows, macrophages help remodel the tissue in a controlled way. Exactly why this mechanism is sometimes deactivated is difficult to say, but perhaps it happens when there is at a certain, genetically determined, degree of adiposity," she says.
"It is our hope that these results ultimately lead to new strategies for preventing or treating type 2 diabetes. It is also conceivable that certain collagen fragments could serve as measurable biological markers, for example, to identify individuals at higher risk of developing type 2 diabetes."
More information: Milica Vujičić et al, A macrophage-collagen fragment axis mediates subcutaneous adipose tissue remodeling in mice, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2313185121