This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
How a natural protein can help fight Alzheimer's disease
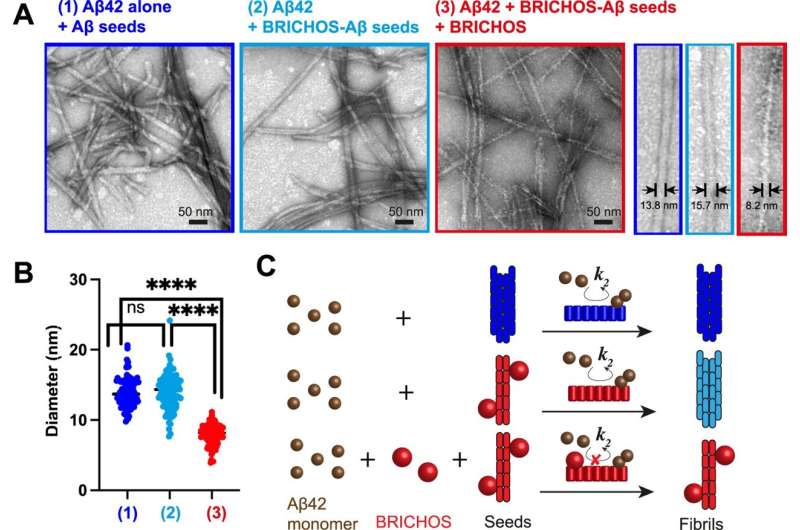
A new study published in Nature Communications gives insights into the underlying mechanisms of the formation of protein clumps in Alzheimer's disease. The study, led by researchers from Karolinska Institutet, could pave the way for new treatments for this devastating neurodegenerative disorder.
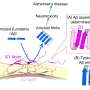
Alzheimer's disease affects millions of people worldwide, causing memory loss, confusion, and cognitive decline. One of the main features of the disease is the accumulation of abnormal protein clumps, called amyloid fibrils and plaques, in the brain. These clumps interfere with the normal functioning of brain cells and may trigger inflammation and cell death.
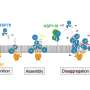
Scientists have been trying to understand how these fibrils form and how to prevent their formation. One strategy is to use proteins that can bind to specific sites on the surface of the fibrils and block the generation of new aggregates. These proteins are called molecular chaperones, and they are naturally produced by cells to help other proteins fold and function properly.
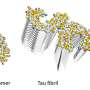
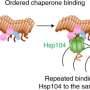
"In our study, we used a specific molecular chaperone, called BRICHOS," says Axel Abelein, last author of the study. "BRICHOS has previously been shown to inhibit the formation of amyloid fibrils. Now, we wanted to find out how it recognizes and binds to the surface of the fibrils, giving us hints on which parts of the fibrils new aggregates are produced."
Targeting hotspots
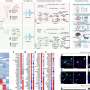
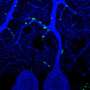
The researchers at the Laboratory for Protein Misfolding and Assembly, Department of Biosciences and Nutrition, Karolinska Institutet, used advanced structural biology techniques, such as solid-state nuclear magnetic resonance (NMR) and electron microscopy, to visualize the structure and interactions of BRICHOS and the fibrils at the atomic level.
They discovered that BRICHOS can sense and attach to specific regions on the fibrils, which may act as aggregation hotspots. By binding to these hotspots, BRICHOS can likely prevent further generation of aggregates and thereby suppress their toxic effects.
The researchers suggest that targeting these aggregation hotspots could be a promising way to interfere with the fibril formation process and its harmful effects in Alzheimer's disease. They also plan to investigate whether similar mechanisms are involved in other neurodegenerative disorders, such as Parkinson's disease, that are also characterized by protein aggregation.
This study was performed in collaboration with groups in Lyon, France and Riga, Latvia, which provided access and expertise to new solid-state NMR instrumentation.
More information: Rakesh Kumar et al, Identification of potential aggregation hotspots on Aβ42 fibrils blocked by the anti-amyloid chaperone-like BRICHOS domain, Nature Communications (2024). DOI: 10.1038/s41467-024-45192-4