This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Enhancing hypoxia-activated prodrug anticancer therapy
Hypoxia-activated prodrugs (HAPs) have the potential to activate specifically in hypoxic tumors and eliminate tumor cells, which has brought new opportunities for safe and effective anticancer therapy. However, their therapeutic efficacy is limited by inadequate hypoxia within the treated tumors.
What is more important, inadequate hypoxia in tumors means that the difference in oxygen levels between tumors and normal tissues is not significant. The insignificant difference leads to HAPs with a decreased targeting ability to tumors, insufficient activation in tumors, and weakened cytotoxicity to tumor cells, severely impacting anticancer treatment efficacy.
Although hypoxia stratification of patients may improve the treatment efficacy of HAPs, this approach would greatly limit the scope of HAPs treatment. Like most HAPs, tirapazamine (TPZ) has been approved for multiple clinical trials, relying on its excellent anticancer potential. However, TPZ did not exhibit survival advantages in phase III clinical trials due to the insufficient hypoxia levels in patient's tumors.
In a new research article published in the Beijing-based National Science Review, scientists at Changchun Institute of Applied Chemistry and University of Science and Technology of China present a new universal well-rounded nanosystem with amplified active targeting, enhanced drug activation and increased hypoxic cytotoxicity to enhance the anticancer efficacy of TPZ.
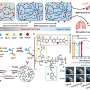
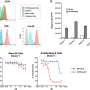
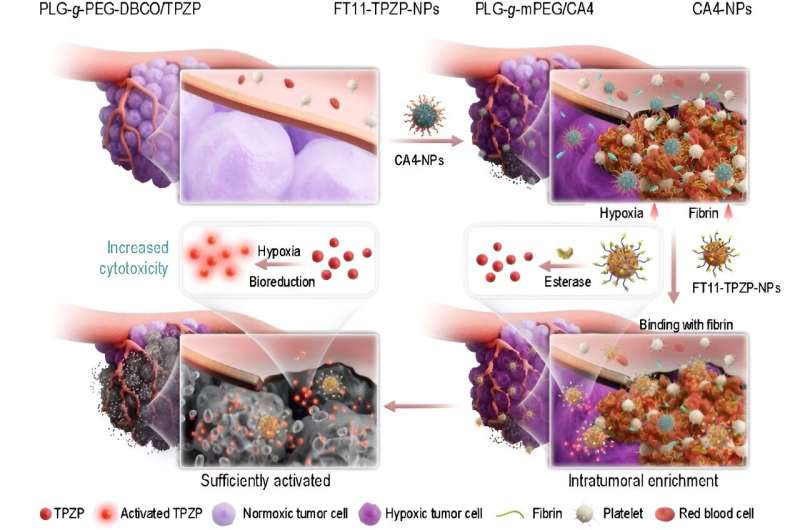
It's composed of two indispensable modules: a tumor microenvironment remodeling nanodrug (CA4-NPs) and a nanodrug of the cytotoxicity-enhanced TPZ derivative (FT11-TPZP-NPs). To improve TPZ's cytotoxicity, the authors first introduced urea to synthesize a series of urea-containing derivatives of TPZ. All urea-containing TPZ derivatives showed increased hypoxic cytotoxicity (9.51~30.85-fold) compared to TPZ while maintaining hypoxic selectivity.
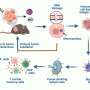
TPZP, one of these derivatives, showed 20-fold higher cytotoxicity than TPZ while maintaining a similar hypoxic cytotoxicity ratio (HCR). To highly efficiently deliver TPZP to the tumors and reduce its side effects in normal tissues, the authors further prepared TPZP into a nano drug with the fibrin-targeting ability (FT11-TPZP-NPs). CA4-NPs, a vascular disrupting agent, was used to remodel the tumor microenvironment with increased fibrin levels within tumors for amplified active targeting and exacerbate tumor hypoxia for sufficient drug activation.
By combining with CA4-NPs, FT11-TPZP-NPs can enrich abundantly in the hypoxia-aggravated tumors and activate sufficiently to kill tumor cells. Following a single-dose administration, FT11-TPZP-NPs + CA4-NPs demonstrated a substantial inhibition rate of 98.1% against CT26 tumor models with an initial volume approximating 480 mm3.
Furthermore, this treatment resulted in the complete eradication of 66.7% of tumors, exhibiting a significant antitumor effect. This study introduces a novel strategy for enhancing the anticancer efficacy of TPZ and other HAPs.
More information: Yajun Xu et al, Introducing urea into tirapazamine derivatives to enhance anticancer therapy, National Science Review (2024). DOI: 10.1093/nsr/nwae038