This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Key mechanism governing bone marrow stem cells opens door to new therapies
A key mechanism controlling how bone marrow stem cells work has been revealed in a new study, shining a light on the principles of stem cell biology and opening the door to new therapeutic pathways.
Eric So, Professor and Chair in Leukaemia Biology in the Comprehensive Cancer Centre, and his lab identified two molecules that control when bone marrow stem cells rest and recover, and when they spring into action and replicate. The researchers subsequently discovered an enzyme that mediates the functions of these molecules. Since bone marrow stem cell transplantation has been the key treatment for a large array of blood cancers, their findings open the door to a new pathway for their future therapeutics.
The research is published in the Blood Journal.
"Given the critical functions of stem cells in bone marrow transplant and cancer biology, identification of a new druggable pathway not only will help to better understand the stem cell biology but also facilitates the development of more effective therapeutics in the future," said So.
Life-long replenishment for the blood system is dependent on hematopoietic stem cells (HSCs), a rare population of self-renewing stem cells that mainly live in bone marrow. HSCs are key, because when needed, they can turn into different blood cells, such as red blood cells, white blood cells and platelets.
HSCs have two statuses: inactive and active. When they are inactive, a status also known as quiescent, they are protected from environmental stressors and are able to rest, preventing exhaustion. However, inactive HSCs must become active again to replicate themselves to replenish the blood system in response to problems such as infection, blood loss and other complications.
There is a sophisticated and delicate balance to keeping stem cells inactive and protected, but at the same time also allow them to respond to stress when needed and replicate themselves. This balance plays a key role for bone marrow transplantation—a life-saving procedure for various devastating diseases such as cancer. Similarly, the balance is also critical for cancer stem cells that sustain the disease and cause relapse. It is therefore critical to understand this process in order to design better treatments.
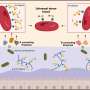
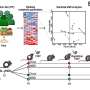
In the study, Professor So and his team found that two important molecules, namely Hoxa9 and b-catenin, work closely together to safeguard both these features in controlling the inactive and active statuses of HSCs. The team learned that one molecule is able to compensate for the other when it is inactive, providing a resilient system to protect our blood production.
These findings already help researchers understand to a greater extent the key principles involved in stem cell biology. However, Professor So and his lab subsequently identified a critical enzyme named PRMT1 which mediates the function of Hoxa9 and b-catenin. Doctors already possess the ability to modulate PRMT1 in clinics, which means that understanding this biological process could provide new avenues for the development of efficient stem cell therapeutics.
More information: Jennifer Lynch et al, Hematopoietic stem cell quiescence and DNA replication dynamics maintained by the resilient β-catenin/Hoxa9/Prmt1 axis, Blood Journal (2024). DOI: 10.1182/blood.2023022082