This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
New type of cerebellar ataxia discovered: How the immune system destroys the cerebellum
Cerebellar ataxia is a neurological disorder of the cerebellum. This important area at the back of the brain acts as a conductor, so to speak, coordinating our movements and keeping us in balance.
This ability is impaired in cerebellar ataxia. Affected people can have difficulties walking, speaking and grasping or even with controlled eye movements. In some cases, the damage begins gradually and develops over a period of years. It can have various causes, which are often genetic. However, strokes or tumors can also be triggers.
A research team led by Professor Dr. Kurt-Wolfram Sühs, Senior Physician at the Department of Neurology with Clinical Neurophysiology at Hannover Medical School (MHH), has now discovered a new type of cerebellar ataxia. This is caused by a specific autoantibody and, in contrast to the previously known subgroups, progresses very quickly.
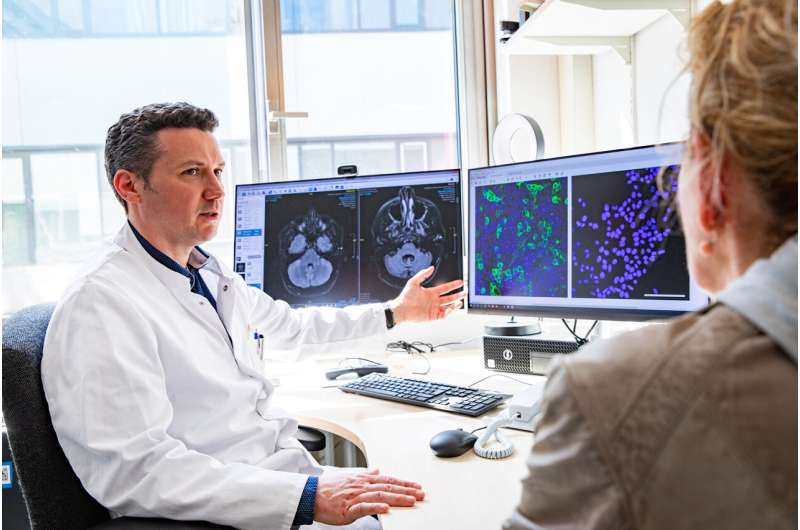
The autoantibody (anti-DAGLA) is directed against cerebellar cells and thus leads to severe inflammation with the corresponding symptoms. The researchers discovered it in the cerebrospinal fluid of four patients between the ages of 18 and 34 who suffered from pronounced gait, speech and vision disorders. Magnetic resonance imaging (MRI) examinations showed a significant loss of substance in the affected cerebellum.
After treatment with anti-inflammatory drugs and immunotherapy with the active ingredient rituximab, which has been used successfully for several years to treat autoimmune diseases, three of the four patients showed a lasting improvement in their state of health. The results of the study, which was conducted in collaboration with other clinics in Belgium, Germany, Luxembourg and Austria, have been published in the Journal of Neurology, Neurosurgery & Psychiatry.
Rapid progression of the disease
"The four people affected were independent and healthy before the onset of the disease," says Professor Sühs. The neurologists found a very high number of defense cells in the cerebrospinal fluid in all of them, which actually indicates a bacterial or viral infection. This was also supported by the rapid progression of the disease—the 18-year-old patient, for example, showed severe symptoms within two weeks, saw double vision and had significant movement disorders.
"However, as we could not detect any bacteria or viruses, we had blood serum and cerebrospinal fluid analyzed in the MHH's own cerebrospinal fluid laboratory," adds the neurologist.
This led to the suspicion of an autoantibody, and the anti-DAGLA autoantibodies responsible for the destruction of the nerve cells in the cerebellum were ultimately discovered as part of the joint research work.
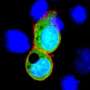
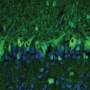
The researchers provided one of the decisive pieces of evidence by loading viral gene shuttles with the blueprint for the DAGLA protein and placing them in cell culture. In the cells, the gene shuttles unloaded their blueprint, the cells converted it and produced the DAGLA protein on their surface. The samples from the cerebrospinal fluid and blood serum of the four patients were then placed on these cell cultures.
The researchers found that where the cells had produced the protein on their surface, the anti-DAGLA autoantibody docked, which could be visualized in a special staining pattern under a fluorescence microscope. This indirect immunofluorescence method is considered the standard technique for the detection of autoantibodies.
In contrast, comparison with samples of blood serum and cerebrospinal fluid from healthy people and patients with other neurological diseases revealed either no binding activity at all, as no anti-DAGLA antibodies were present in the cerebrospinal fluid. Or their antibodies docked to a different region of the DAGLA protein.
"This means that the anti-DAGLA autoantibody we discovered binds highly specifically and is therefore suitable as a biomarker for this form of cerebellar ataxia," explains Professor Sühs.
Early detection crucial for diagnosis and therapy
"Until the tests are established in routine practice, however, it could be difficult to distinguish between these two groups of anti-DAGLA autoantibodies," Professor Sühs suspects. For diagnostic purposes, he therefore recommends that only those anti-DAGLA autoantibodies that have been detected in the cerebrospinal fluid with corresponding signs of the disease should be considered as markers for a new form of progressive cerebellitis.
"However, the early detection of anti-DAGLA autoantibodies in the cerebrospinal fluid can be decisive for the diagnosis of this rapidly progressive cerebellitis and the initiation of immediate treatment," emphasizes the neurologist.
As the cohort was very small with only four patients suffering from this new form, further studies with more patients are needed to verify the diagnostic significance of anti-DAGLA autoantibodies and to optimize treatment recommendations. For example, it is still unclear whether the autoantibodies dock to the surface and what molecular mechanisms they trigger—whether they shut down the protein itself or whether they signal other immune cells to destroy the affected cerebellar cell through the connection.
"So far, we have only been able to suppress the autoimmune reaction in a relatively untargeted way, for example by removing the autoantibodies from the body by blood washing or by using the drug rituximab to destroy the B cells of the immune system, which are responsible for the formation of the autoantibodies once they have matured," says Professor Sühs.
The aim is to develop a therapy that only targets the pathogenic cells of the immune defense that actually form the anti-DAGLA autoantibodies and destroy the cerebellar cells.
More information: Ramona Miske et al, Identification of DAGLA as an autoantibody target in cerebellar ataxia, Journal of Neurology, Neurosurgery & Psychiatry (2024). DOI: 10.1136/jnnp-2024-333458