This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Towards molecular and phenotypic characterization of VEXAS syndrome

VEXAS syndrome is a rare, adult-onset, life-threatening autoinflammatory disease caused by a genetic mutation. The pathophysiology is still unknown, but new work presented at the 2024 congress of EULAR—The European Alliance of Associations for Rheumatology—aims to provide a molecular and phenotypic characterization of hematopoiesis in VEXAS patients, and to develop cellular and humanized mouse models by gene editing.
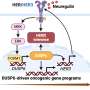
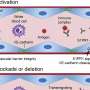
VEXAS is characterized by predominantly rheumatic and hematologic systemic involvement, and caused by somatic mutation in UBA1—a gene encoding ubiquitin-activating enzyme 1, which is necessary for a post-translation modification that affects protein functions ranging from degradation to subcellular localization and kinase activation. The syndrome was first described in 2020, but diagnosis can be challenging as the symptoms overlap with many other inflammatory conditions. Hot on the heels of this recent discovery, research is underway to better understand pathogenesis, clinical features, and potential treatment options.
To support this, 6 patients were recruited from the IRCCS San Raffaele Hospital in Milan, Italy. The variant allele frequency of UBA1 mutant cells was quantified, and multiparametric immunophenotypic analysis and single cell RNA sequencing were performed on peripheral blood and bone marrow, focusing on hematopoietic stem/progenitor cells (HSPC). The results were compared to those from healthy age- and sex-matched controls. Additionally, to introduce UBA1 mutations and develop VEXAS models in healthy human HSPC, the group used cutting-edge gene-editing technologies.
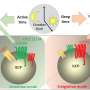
Targeted sequencing supported a myeloid skewing of mutant HSPC in VEXAS patients, and multiparametric immunophenotypic analyses showed unbalanced HSPC composition in bone marrow, with 2- to 3-fold reduction of primitive stem cells, multipotent, and lymphoid progenitors—and 2-fold increase in myeloid progenitors—compared to matched healthy individuals. HSPC, myeloid-biased HSPC, and immature myeloid cells were increased by 3- to 4-fold in the circulation.
Gene expression analysis of circulating monocytes displayed upregulation of inflammatory pathways and metabolic rewiring. Further metabolomic analyses confirmed hyperactivation of the glycolytic pathway and specific lipid metabolism.
The results from the single-cell RNA sequencing of bone marrow mononuclear cells identified a subpopulation of CD34+ cells specific to VEXAS patients—and revealed upregulated stress response and immune activation pathways across VEXAS cell clusters as compared to healthy controls.
In the VEXAS models, transplantation of gene-edited HSPC in immunodeficient mice resulted in a 100-fold reduction in circulating B cells, while NK and myeloid compartments were preserved.
The group conclude that mutations in UBA1 drive expansion of HSPC and enhance myelopoiesis-guided accumulation of myeloid precursors. The success of the new gene-editing models holds promise, and could be used in preclinical testing and validation of novel therapeutics for this rare disease.
More information: C. Campochiaro et al, OP0073 Unraveling pathophysiology and hematopoiesis of VEXAS syndrome by multi-omics analysis and targeted gene editing, Scientific Abstracts (2024). DOI: 10.1136/annrheumdis-2024-eular.3532