This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A modified peptide shows promise for fighting tumors
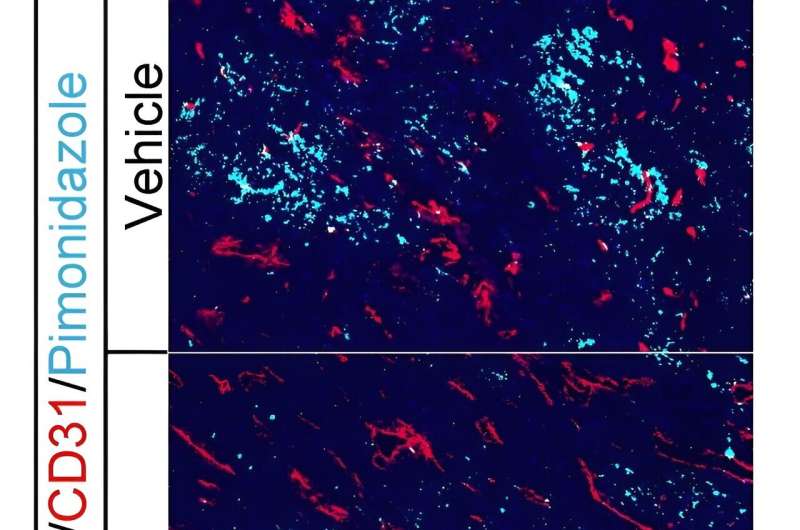
The growth of healthy tissues in the body depends on the development of new blood vessels, a process called angiogenesis, that enables proper blood flow, meaning nutrients and oxygen are delivered while toxic metabolic products are removed.
But solid tumors grow faster than healthy tissues, resulting in deficiencies in oxygen and blood flow, which leads to accelerated formation of dysfunctional blood vessels. Malignant cells rapidly grow while antitumor immune cells quickly lose their viability and function.
These events, cell biologist Serge Fuchs of the School of Veterinary Medicine says, promote generation of the immunosuppressive tumor microenvironment, which stimulates the spread and growth of tumors and confers resistance to antitumor therapies.
Past research has shown how native C-type natriuretic peptide (CNP), a 22-amino acid peptide produced by endothelial cells and fibroblasts, stimulates growth of normal blood vessels and restores proper blood flow and oxygenation within tissues of rodent limbs that weren't getting enough blood flow.
Given the importance of CNP in angiogenesis, researchers reasoned that CNP would also play a critical role in regulating tumor vasculature. But the therapeutic potential of CNP is severely hampered by its short half-life of less than three minutes, says Zhen Lu, a former senior research investigator in Fuchs' lab.
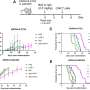
Fuchs and Lu are part of an interdisciplinary, collaborative team which found that modifying CNP stimulated the formation of blood vessels, increased blood flow through tissue, reinvigorated antitumor immune responses, and slowed growth of tumors in an animal model.
The results published in the journal Science Translational Medicine suggest that the treatment could alleviate hypoxia, or insufficient oxygen levels, in tumors. The team includes researchers from Kyushu University, the Higashiosaka City Medical Center, the Case Western Reserve University School of Medicine, and PharmaIN Corp.
"It is a successful approach to target the immunosuppressive tumor microenvironment, which should lead to a breakthrough in treatment of a large variety of solid tumors," says Fuchs, a corresponding author on the paper.
This approach not only elicited responses on its own against solid tumors and metastatic disease but also enhanced the efficacy of multiple therapies. That includes different chemotherapy treatments, radiotherapy, immune checkpoint blockade, and adoptive cell transfer therapies, such as CAR T therapy.
Lu, first author on the paper, explains that PharmaIN researchers chemically modified part of CNP by adding an extended amino acid tail, forming a derivative of CNP (dCNP). He notes that, compared to native CNP, the derivative showed improved pharmacokinetics and pharmacodynamics in mice, meaning it was better absorbed in the body and had better effects.
The researchers successfully tested dCNP in several mouse solid tumor models, including pancreatic, colon, lung, and mammary adenocarcinomas, along with hepatocellular carcinoma and osteosarcoma.
Fuchs says PharmaIN generated the dCNP treatment, researchers from Kyushu University and the Higashiosaka City Medical Center in Japan identified its antitumor effects, and scientists from Penn Vet, the Perelman School of Medicine, and the School of Arts & Sciences examined the mechanism of action.
Fuchs explains that this research builds on the work of Judah Folkman and Rakesh Jain that looked at vascular endothelial growth factor (VEGF), a signaling protein that accelerates the formation of dilapidated and leaky blood vessels. They proposed anti-VEGF therapy. But resistance to anti-VEGF agents and difficulties with precise dosing meant obstacles remained to solving the problem of the immunosuppressive tumor microenvironment.
Lu says the new study demonstrates that, instead of inhibiting dysfunctional tumor angiogenesis by anti-VEGF agents, inducing healthy vasculature by dCNP could reinvigorate anti-tumor immunity and improve solid tumor therapies.
"This study proposed a new paradigm for anti-angiogenic therapy and provided opportunity for developing new anti-angiogenic medication in the future," he says.
Fuchs says researchers completed preclinical studies and PharmaIN completed toxicity profiles, so the next step is selecting a solid tumor model for clinical trials.
More information: Zhen Lu et al, Modified C-type natriuretic peptide normalizes tumor vasculature, reinvigorates antitumor immunity, and improves solid tumor therapies, Science Translational Medicine (2024). DOI: 10.1126/scitranslmed.adn0904