This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New work validates targets for personalized cancer immunotherapy
What are the characteristics of a cancer cell that are recognized by the immune system? Knowledge of the potential target structures for the immune cells is a basic prerequisite for the development of personalized cancer immunotherapies.
Scientists from the German Cancer Research Center (DKFZ) and the NCT Heidelberg have developed a highly sensitive method based on mass spectroscopy to identify such tumor-specific "neoepitopes." The analytical method is designed to detect these low abundance protein fragments and requires minimal amounts of sample material.
The findings are published in the journal Molecular & Cellular Proteomics.
Personalized immunotherapies are considered a promising approach to fighting cancer more effectively. Personalized immunotherapies include therapeutic cancer vaccinations or cellular therapies with T cells whose receptors are tailored to the individual tumor. There is one basic prerequisite for the development of all personalized immunotherapies: The cancer-typically altered protein characteristics by which the patient's immune system recognizes the cancer cells must be known.
Researchers refer to mutated fragments of proteins that are recognized by the immune system as "neoepitopes." In order to detect them, the tumor genome must first be sequenced. Using powerful bioinformatics, the DNA and RNA sequencing data can then be used to detect those mutations that lead to altered proteins and can therefore theoretically be recognized as "foreign" by the patient's immune system.
However, in order to activate the immune system, fragments of the altered proteins must first be presented on the surface of the tumor cells.
"Only those neoepitopes that are presented by the so-called HLA proteins on the membrane of the cancer cells can activate T cells," explains Angelika Riemer, immunologist at the DKFZ.
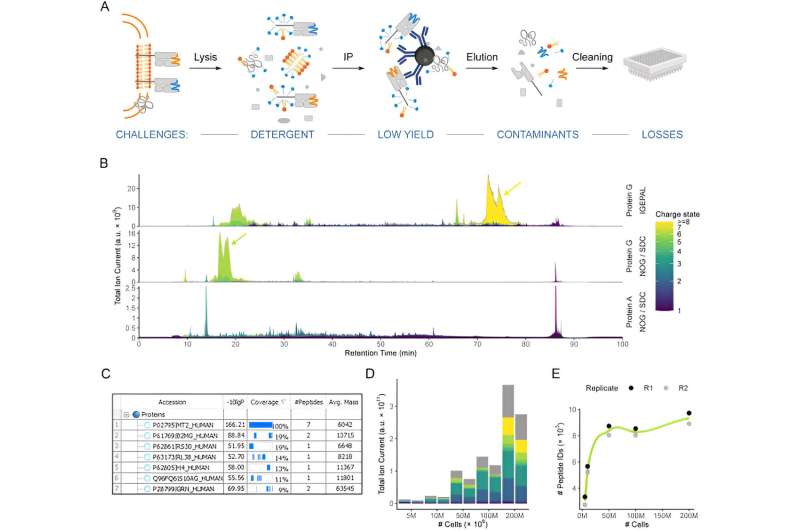
Mass spectrometry (MS) is used to detect and identify such neoepitopes. This analysis method is based on the determination of the mass of electrically charged protein fragments.
"MS provides the only real proof that a neoepitope is actually presented. However, with standard MS methods, low abundance peptides such as tumor neoepitopes are often lost and not detected," explains the researcher.
Riemer and colleagues from the DKFZ and the NCT Heidelberg have now published an analytical method to determine the individual cancer neoepitopes of patients faster and more precisely in the future.
Using the sequences of tumor DNA and RNA, the researchers first narrowed down the protein fragments in question. Precise knowledge of the binding properties of the HLA molecules also helps to predict which neoepitope is most likely to be presented on the tumor surface.
Now comes a trick: These peptides are first synthesized in the laboratory and used to optimize the analysis settings of the mass spectrometer for each individual peptide. Only then is a real tumor tissue sample measured. The researchers now know exactly the device settings under which the neoepitopes can best be detected.
"As a result, the new protocol means that much smaller tumor tissue samples are sufficient for the measurement," explains Riemer. Her team has succeeded in detecting a neoepitope in a sample of just two and a half million cancer cells. "That's not even the volume of a grain of sand," explains the immunologist.
The team was able to detect a total of five neoepitopes in small tumor tissue samples from three patients, and in some cases even confirm them immunologically through the reaction of the patients' T cells.
"Personalized cancer immunotherapies will play an increasingly important role in the future," says senior author Riemer. "In this context, MS provides the ultimate proof that a neoepitope is presented on the surface of cancer cells—and is therefore a worthwhile therapeutic target. Our optiPRM protocol will help to provide this evidence from minimal tissue samples and suggest validated tumor epitopes to clinicians for individualized cancer therapy."
The mRNA-based tumor vaccines currently undergoing clinical trials often contain around 30 different predicted cancer neoepitopes. Riemer concludes, "We believe that a targeted approach with validated neoepitopes could achieve the same efficacy with significantly fewer epitopes."
Even more important, the experts emphasize, is the validation of the target epitopes for the development of therapeutic T cells that are equipped with a specific receptor to specifically attack cancer cells.
More information: Mogjiborahman Salek et al, optiPRM: A targeted immunopeptidomics LC-MS workflow with ultra-high sensitivity for the detection of mutation-derived tumor neoepitopes from limited input material, Molecular & Cellular Proteomics (2024). DOI: 10.1016/j.mcpro.2024.100825