New models of drug-resistant breast cancer hint at better treatments
Breast cancer that spreads to other organs is extremely difficult to treat. Doctors can buy patients time, but a cure remains elusive. Now, researchers at Washington University School of Medicine in St. Louis have shown that human breast tumors transplanted into mice are excellent models of metastatic cancer and could be valuable tools in the search for better treatments.
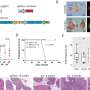
According to new research published Sept. 19 in Cell Reports, these transplanted tumors maintain the genetic errors that caused the original cancer, even though they are growing in mice. As such, mice carrying human tumors can help identify drivers of tumor growth and serve as excellent test subjects for investigating new drugs.
Senior author Matthew J. Ellis, MD, PhD, said the new paper is a step toward precision medicine, allowing researchers to study model tumors matched to a specific patient whose treatment regimens are well-documented.
"It is so powerful to have a model of Mrs. Jones' cancer or Mrs. Smith's cancer," said Ellis, a breast cancer specialist who treats patients at Siteman Cancer Center at Barnes-Jewish Hospital and Washington University. "First, we have carefully documented information about the patient. We know exactly what drugs she responded to and what drugs she didn't. Second, we have her consent for full genetic analysis. And third, we take the cells from her cancer and grow them in a special strain of mice that has no immune system, so they grow cells from a human without rejection."
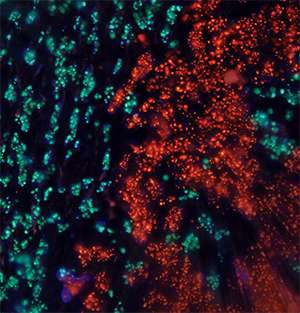
Ellis and his colleagues reported that the breast tumors growing in the mouse are startlingly similar to cancers growing in the human. And the changes that do occur in the transfer from human to mouse are often "silent," having no effect on tumor growth.
"We are addressing a fundamental question about how similar or different the tumor is once it's in a mouse," said Elaine Mardis, PhD, co-director of The Genome Institute, which performed the sequencing. "These results give us confidence that pursuing new therapies using the mouse will provide an accurate picture of how that therapy will impact the tumor in the patient."
The analysis showed that mutations occurring at high frequency in the original tumor remained at high frequency in the mouse. Likewise, and somewhat surprisingly, according to Ellis, less common mutations remained at the same low frequency in the mouse.
"A simple growth model says the slower growing clones should go away," Ellis said. "They shouldn't be maintained at the same low frequency, but they are. And this drive for equilibrium is so strong it can survive crossing a species barrier. In maintaining this hierarchy, the genetically different clones in the tumor are interacting, a bit like different cell types within an organ, which is a wild thought because it means that our old models based on competition between the clones may be incorrect."
Ellis emphasized that this approach is fundamentally different from most breast cancer research. Others have grown human tumors in mice, but Ellis' team is the first to sequence whole genomes—the patient's healthy genome, tumor genome and the corresponding mouse genome—to determine how closely the mouse tumor resembles that in the human. Others have tested new drugs in breast cancer cell lines growing in a dish, but these cell lines are far removed from any information about the original patient the cells were taken from and how that patient fared with a particular kind of treatment.
"We are trying to close the gap between what is happening in the clinic and what goes on in the laboratory," said Ellis. "We learned that our system was mostly capturing rapidly growing, treatment-resistant lethal tumors, which is exactly what our studies should focus on."
The approach also allowed the researchers to identify new mutations that appeared to be driving the strong drug resistance exhibited by these tumors. Specifically, they found mutations in the estrogen receptor.
"Research over the past 20 years has shown tantalizing hints that patients whose disease stops responding to anti-hormonal agents have changes in the estrogen receptor," said Ellis. "And we found all three types of 'gain-of-function' mutations in the estrogen receptor gene ESR1 in the tumor samples."
This study focused on estrogen receptor (ER) positive breast cancer—the most common type—that also is resistant to standard treatment. Unlike ER positive cancers that respond well to treatment, those that are drug resistant spread elsewhere in the body even with aggressive treatment.
Typically, ER positive tumor growth is driven by the presence of estrogen. Block or remove the estrogen with different types of drugs, such as the commonly prescribed tamoxifen or aromatase inhibitors, and the tumor stops growing. Some women with estrogen receptor positive breast cancer do extremely well on such anti-hormone treatment. But some don't, and it's not clear why.
Perhaps shedding light on this mystery, the researchers found three different types of mutations in the estrogen receptor in patients whose cancer was resistant to anti-hormone therapy. One type of mutation is called gene amplification, in which multiple copies of the ESR1 gene are present. A second type is point mutations in the part of the receptor that binds estrogen, causing the receptor to become active even without estrogen. And the third type was a translocation, in which half of the estrogen receptor gene was swapped for a completely unrelated gene from a different part of the genome.
Similar to the way breast cancer patients currently are told whether their tumors make estrogen receptor, Ellis envisions a clinical test that could tell a patient whether and how the estrogen receptor is mutated.
"We want to be able to make a diagnosis based on the specific tumor biology," Ellis said. "For example, if we look at ESR1 and see amplification or point mutations or a translocation, we want to design clinical trials to determine whether we should give drug A, drug B or drug C.
"Our results are a good start for designing cures for metastatic breast cancer, which is a long-term goal for many of us who work with patients with stage 4 disease."
More information: Li S, et al. Endocrine therapy resistant ESR1 variants revealed by genomic characterization of breast cancer derived xenografts. Cell Reports. Sept. 19, 2013.