New insight in quest for single vaccine against multiple influenza strains
(Medical Xpress)—A study led by St. Jude Children's Research Hospital scientists highlights a new approach for developing a universal influenza vaccine that could protect against multiple flu strains, including deadly pandemic strains. The research appears today in the advance online edition of the scientific journal Nature Immunology.
Researchers used the immune suppressing drug rapamycin to shift the immune response following flu vaccination to favor production of antibodies that broadly target flu viruses. The result was a more diverse antibody response to the vaccination that expanded protection to include pandemic strains not targeted by the vaccine. Vaccination is the most effective strategy against flu, particularly the pandemic strains that emerge periodically, but efforts to develop a single, universal vaccine against all flu strains have been unsuccessful.
The findings highlight a novel way to generate antibodies that recognize and target proteins shared by most influenza A strains rather than those unique to each strain. Antibodies are produced by B cells to recognize and defend against viruses. The same strategy might aid efforts to design vaccines against other viruses, researchers said.
Current flu vaccines emphasize production of highly specific antibodies. They target and bind tightly to strain-specific regions of hemagglutinin (HA) and neuraminidase (NA) proteins on the virus. That approach requires developing and administering a new flu vaccine each year to keep up with changes in those unique and highly variable HA and NA proteins.
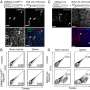
Investigators showed the new strategy protected mice – vaccinated against the H3N2 influenza A flu strain, which causes mild disease – from succumbing to the more dangerous H5N1 and H7N9 strains weeks later. When researchers transferred antibody-rich serum from vaccinated to unvaccinated mice, the unvaccinated animals were also protected from later H5N1 infection, an indication that the protection came from antibodies rather than from other immune system components.
"This study has changed our approach to developing a universal flu vaccine," said corresponding author Maureen McGargill, Ph.D., an assistant member of the St. Jude Department of Immunology. "Instead of trying to enhance a highly specific, targeted immune response, our results show that a more diverse, less focused response provides a broader repertoire of antibodies that target different flu strains."
Influenza – particularly pandemic strains that emerge periodically as flu viruses mix and form novel strains – remains a global health threat. The influenza A H5N1 avian pandemic strain has a mortality rate of nearly 60 percent. The World Health Organization estimates that each year flu and flu-related complications kill more than 250,000 individuals worldwide. Vaccination is the most effective strategy to combat the infection. But existing vaccines protect against just the dominant seasonal flu strain and not emerging flu strains.
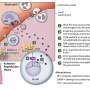
This study also advanced understanding of the role a protein named mTOR plays in generating the highly specific antibodies. Rapamycin works by inhibiting mTOR, which is involved in cell survival and proliferation. Researchers used the drug to track how blocking mTOR affected the immune response of mice following H3N2 vaccination.
Inhibiting mTOR disrupted generation of the antibodies that target specific regions of the HA proteins that are unique to each flu strain. Researchers showed that loss of mTOR delayed the formation of the immune structure called a germinal center. That is where antibodies are reshaped through a process called class switching. The process hones their focus and primes them to target flu viruses based on the unique, rather than shared, surface proteins.
The finding was surprising because previous research had highlighted a likely role for white blood cells known as CD8+ and CD4+ memory T cells for broadening the immune response against different flu strains. Unlike antibodies, the T cells recognize flu viruses based on shared internal proteins. The T cells reduce flu-related complications by eliminating flu-infected cells and speeding the virus' clearance from the body. In addition, rapamycin was known to increase the number of memory CD8+ T cells.
McGargill and her colleagues showed that memory CD8+ T cells were not required for enhanced protection in rapamycin-treated mice following vaccination and that the CD4+ cells played an indirect role. "This led us to the B-cell response and evidence that the cross-reactive antibodies provide crucial protection against different flu strains," said first author Rachael Keating, Ph.D., a St. Jude scientist.
The other authors are Tomer Hertz, Zachary Wilson and Philip Bradley, all of Fred Hutchinson Cancer Research Center, Seattle; and Marie Wehenkel, Tarsha Harris, Benjamin Edwards, Jennifer McClaren, Scott Brown, Sherri Surman, Julia Hurwitz, Hongbo Chi, Peter Doherty and Paul Thomas, all of St. Jude.
More information: Paper: dx.doi.org/10.1038/ni.2741