A new neuron repair pathway could one day help people with nerve damage

(Medical Xpress)—Other parts of your body—such as skin and bone—can be replaced by the body growing new cells, but when you injure your neurons, you can't just grow new ones; instead, the existing cells have to repair themselves.
In the case of axon injury, the neuron is able to repair or sometimes even fully regenerate its axon. But neurons have two sides—the axon (which sends signals to other cells) and the dendrite (which receives signals from other cells).
Melissa Rolls, an associate professor of biochemistry and molecular biology at Penn State and director of the Huck Institutes' Center for Cellular Dynamics, has done extensive comparisons of axons and dendrites—culminating recently in a paper published in Cell Reports.
"We know that the axon side can repair itself," says Rolls, "and we know a bunch of the molecular players. But we really didn't know whether neurons have the same capacity to regenerate their dendrites, and so that's what we set out to find in this study."
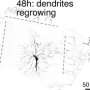
"Our lab uses a Drosophila model system, where the dendrites are very accessible to manipulation," she says, "so we decided that we would start by removing all the dendrites from the neurons to see if they could regenerate. We didn't start with anything subtle, like taking off just a few dendrites. We said 'Let's just push the system to its maximum and see if this is even possible.' And we were surprised because we found that not only is it possible, it's actually much faster than axon regeneration: at least in the cells that we're using, axon regeneration takes a day or two to initiate, while dendrite regeneration typically initiates within four to six hours and it works really well. All the cells where we removed the dendrites grew new dendrites – none of them died; so it's clear that these cells have a way to both detect dendrite injury and initiate regrowth of the injured part."
Further inquiry
Having confirmed the neurons' ability to initiate dendrite regeneration, the researchers then set out to find the injury surveillance system used by the cells to detect dendrite injury.
"We wanted to know," says Rolls, "whether these cells were using the same kind of injury surveillance system for both the axons and the dendrites. In the case of axon injury, there's a kinase—a signaling molecule—called DLK that is known to be used to initiate axon regeneration in everything from soil nematodes to Drosophila to mammals. We confirmed that in the cells we were studying, the DLK signaling molecule is required for axon regeneration, and then we did the same experiment to see whether DLK was required for dendrite regeneration—and it turns out that it's not."
Using a technique known as RNA interference (RNAi) to block the synthesis of the DLK kinase, and also by using Drosophila strains where the DLK gene is mutated, the researchers observed that the dendrites regrow normally without DLK. The researchers also used reporter strains to observe activity in the DLK pathway, and various loss-of-function approaches—all of which showed DLK's involvement in axon regeneration, but never in dendrite regeneration.
"Since this paper came out," says Rolls, "we've tested a bunch of other pathways that are known to be required for axon regeneration, and none of them are required for dendrite regeneration—which is kind of cool, because it means that these cells have a way that we didn't even know about to respond to dendrite injury, and it means we essentially know nothing about that process. We don't know how they sense the injury, or what they have to do to regrow dendrites. And so that's something we're now trying to figure out."

Rolls Lab
Rolls—a faculty member of the Huck Institutes' graduate programs in cell and developmental biology, genetics, and neuroscience at Penn State – advises students from each program who also work in her lab and who performed the experiments for this study.
Li Chen, a PhD student in the Cell and Developmental Biology program, is studying the signaling mechanisms involved in axon regeneration and piloted several of the tools used by the Rolls Lab to distinguish axon and dendrite regeneration, including a reporter assay for the DLK pathway.
"Basically," says Chen, "I pioneered an assay to tell us about the activation of proteins in the DLK pathway; the reporter—a fluorescent protein—functions as a tool for us to know whether the proteins are activated or not after the axon is damaged, and my role was to test whether the reporter would work. Now that we've validated the reporter, we can do further assays to compare how cells are able to tell the difference between axon injury and dendrite injury."
Michelle Stone, a PhD student in the Genetics program and the paper's first author, performed the study's initial, foundational experiments—characterizing the process of dendrite regeneration, testing for molecular requirements of regeneration, and confirming the ability of neurons to regenerate in both adult and larval Drosophila.
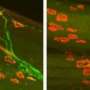
"I did all the initial studies," says Stone, "the first experiments of cutting all the dendrites off our neuron samples to see whether they would regenerate, and then quantitating how they regenerated. I also did experiments in live adult Drosophila, using a pulsed UV laser on a confocal microscope to cut off dendrites—which was a little tricky, because it was hard to keep the adults from getting squashed on the slide—but we were able to see that there was sprouting in those cells where the dendrites had been cut. Then I did the assays and other experiments showing that the DLK pathway was not involved in dendrite regeneration, whereas for axon regeneration it really is required."

Rich Albertson, an MD/PhD student currently completing his doctoral work in the Neuroscience program, was responsible for quantifying the extent of dendrite injury required to initiate regeneration and determining the significance of the injury's proximity to the cell body. Now he is beginning the next phase of the project—identifying the molecular players required for dendrite regeneration.
With the help of a seed grant from the Huck Institutes Core Facilities, Albertson is using the Microscopy and Cytometry Facility's laser-capture microdissection system to sample individual injured neurons; from those neurons, Albertson extracts RNA samples for next-generation sequencing by the Genomics Core Facility.
"In my pilot experiments," says Albertson, "I've been doing a proof of concept using qPCR to investigate effects, at the RNA transcript level, of axon injury. Once we've sequenced the samples, we can align those short reads with the genome and count the total number of reads—which will tell us how many transcripts are present and whether their numbers are going up or down compared to an uninjured control. We'll do this with neurons that have their dendrites cut off, and we'll find genes that are being upregulated or downregulated, and we'll then feed those data into our functional studies and test them to find which candidates are required for dendrite regeneration."
The Rolls Lab has recently launched a new collaboration with a lab at UCLA to investigate the possibility of equivalent or similar dendrite regeneration processes existing in vertebrates—with the hope of finding potential applications in several scenarios: stroke (which involves neuron damage in the central nervous system) and peripheral neuropathy (a loss of sensation in the periphery, often induced by diabetes or chemotherapy).
"We don't yet know in which, if either, of these scenarios dendrite regeneration will happen," says Rolls, "but this is an issue that we really need to resolve. Now that we've found that Drosophila sensory endings don't use the DLK pathway to regenerate, we want to look in the vertebrate system and try to figure it out. In an era when we feel as though we've identified most of the basic biological processes, it's fascinating to find that there's something we don't know, that our hypotheses were all wrong, and that things are, in fact, wide open for exploration."
More information: "Dendrite Injury Triggers DLK-Independent Regeneration." Stone, Michelle C. et al.. Cell Reports , Volume 6 , Issue 2 , 247 - 253. dx.doi.org/10.1016/j.celrep.2013.12.022