How defects in a signaling protein sabotage the immune system in multiple, seemingly contradictory ways
The antibody response to immune threats is managed by cells known as B lymphocytes. The differentiation and function of B cells are tightly regulated to ensure a prompt response to confirmed dangers, such as viruses or bacteria, and also to prevent the emergence of harmful autoimmune responses that can damage healthy tissues in the body.
Many B cells express a protein called TACI on their surface, but its specific function has remained ambiguous. "Human patients with TACI mutations manifest two seemingly opposing conditions—immunodeficiency as well as autoimmunity," says Kong-Peng Lam, a researcher at the A*STAR Bioprocessing Technology Institute. By exploring the roots of these contradictory effects, Lam and his team have provided an explanation.
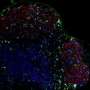
Much of the antibody response is marshaled at sites known as germinal centers (GCs), where so-called 'follicular helper T (Tfh) cells' essentially 'train' GC B cells to respond to infectious threats. Lam and co-workers examined mice that lack TACI, and found that these mice generated considerably greater numbers of both Tfh and GC B cells. A pathway driven by a signaling protein called BAFF plays a major role in driving GC B cell proliferation; the researchers determined that this pathway is hyperactive in TACI-deficient mice, and proposed a model wherein TACI normally sequesters excess BAFF to limit cellular expansion of GCs.
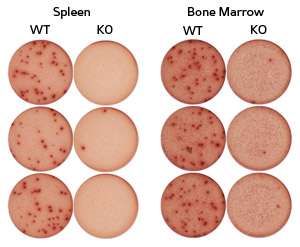
In spite of the increased GC B cells, TACI-deficient mice generally failed to mount an efficient antibody response against an immune challenge. Upon closer examination, Lam and co-workers found that these animals had fewer plasma cells (see image), the class of B lymphocytes that secretes antibodies, and determined that TACI acts as a 'survival factor' that enables these cells to flourish.
Although these two effects would seem to counteract each other, the researchers' proposed model links these physiological changes with the unusual immune defects observed in human patients. Lam notes that B cell selection in the GC also entails elimination of harmful B cells that recognize host antigens. "Absence of TACI increases the population of GC B cells, and this probably has the follow-on effect of reducing the stringency of the selection process, such that autoimmunity may arise," he says.
Thus, while the drop in the number of plasma cells would result in overall immunodeficiency, the 'quality' of surviving plasma cells would also drop. "The few antibody-secreting cells that manage to survive may be autoreactive," says Lam, "and this explains the two seemingly opposing conditions that are found in TACI-deficient patients."
More information: Ou, X., Xu, S. & Lam, K.-P. Deficiency in TNFRSF13B (TACI) expands T-follicular helper and germinal center B cells via increased ICOS-ligand expression but impairs plasma cell survival. Proceedings of the National Academy of Sciences 109, 15401–15406 (2012). www.pnas.org/content/early/201 … /1200386109.abstract