October 29, 2013 feature
Mind over gray matter: Placebo improves both pleasure and pain
(Medical Xpress)—The human brain's exquisite complexity and power make it a unique evolutionary marvel. One of the brain's more interesting abilities is known as the placebo effect, in which no more than the expectation of relief can lead to analgesia – the relief of pain, anxiety, depression, nausea, and many other aversive states. However, scientists at University of Gothenburg and University of Oslo recently showed that the placebo effect may not be limited to pain reduction, but may also enhance pleasure, or hyperhedonia. The researchers used the placebo effect to improve both painful and pleasant touch sensations in healthy humans – and by comparing brain processing using functional magnetic resonance imaging (fMRI), found that, depending on whether the starting point was painful or pleasant, neurocircuitry associated with emotion and reward underpinned improvement of both pain and pleasant touch by dampening pain but increasing touch pleasantness.
In an interview with Medical Xpress, PhD candidate Dan-Mikael Ellingsen discussed the paper he and his colleagues published in Proceedings of the National Academy of Sciences. "In recent years, functional brain imaging studies have shown that expecting a treatment to relieve negative symptoms – like pain, anxiety or unpleasant taste – leads to not only subjective reports of relief, but also suppressed brain activity in sensory circuitry during aversive stimuli, such as noxious heat or touch, threatening images, and unpleasant taste," Ellingsen tells Medical Xpress. "However, both aversive and appetitive experiences – for example, tasty food or a pleasant touch – are affected by context and expectation." Therefore, Ellingsen explains, in forming their hypothesis for this study, the researchers asked whether improvement of good experiences is encoded entirely in higher-level valuation processing, or whether it would mirror the modulation of early stages of sensory processing that is seen for aversive stimuli. "If so, we'd expect such positive sensory signals to be up-regulated, in contrast to the down-regulation of sensory signals we see during placebo-induced reduction of aversive experiences."
In the placebo manipulation procedure, participants were shown a short video documentary convincing them that a nasal spray containing the neuropeptide oxytocin would reduce pain and enhance the pleasantness of pleasant touch. Following this video, they self-administered 10 puffs of a placebo nasal that they were told could contain oxytocin. The pleasant touch stimuli consisted of caress-like light strokes with a soft brush, or a hot/cold pack (resembling a warm hand, applied to the subject's forearm. The pain stimulus was a thermode (~47 degrees Celsius) on the hand.
Ellingsen notes that by comparing brain activation during painful or pleasant touch stimuli after placebo treatment versus no-placebo, the scientists were able to assess differences in activation that was specifically related to having received placebo treatment. "Importantly, the subjective reports showed that, after receiving placebo relative to no-placebo, touch pleasantness was increased while pain unpleasantness was decreased," he adds. "When contrasting placebo and no-placebo on brain activation, we found that sensory activation was increased during pleasant touch stimuli and decreased during painful touch stimuli. In other words, the placebo-induced change in sensory processing reflected the placebo-induced change in subjective reports."
The team also hypothesized that placebo improvement of pleasant touch would recruit the same emotion appraisal neurocircuitry that underpins placebo analgesia. "Neural systems mediating pain and pleasure interact extensively, with pain and pleasure often being mutually inhibitory," Ellingsen says. "For instance," he illustrates, "pleasant stimuli such as music, food, odors, and touch can have analgesic effects – and pain can inhibit pleasure and positive feelings. Further, opioids can induce both potent analgesia and feelings of pleasure." (An opioid is any psychoactive chemical that resembles morphine or other opiate in its pharmacological effects.) Ellingsen points out that previous findings show that relief from pain induces pleasant feelings1,2, and when a normally painful stimulus represents the best possible outcome – that is, when the alternative is even more intense pain3 – it can even become pleasant.
Ellingsen explains that a central element in all placebo effects is that there is an expectation or desire for an improvement, for example, a relief of pain or unpleasantness – and placebo effects have been theorized to arise from a generalized mechanism of reward prediction. This reasoning, he notes, is supported by evidence that placebo responses across modalities – analgesia6, anxiety relief7, and so on – rely on activation of similar neural systems involved in reward and emotion. "In line with this strong link between pleasure and the relief from negative feelings, we hypothesized that improving the pleasantness of an appetitive stimulus would rely on modulatory mechanisms similar to those involved in the improvement of aversive feelings."
A key aspect of the team's research was devising and applying an fMRI crossover study to compare neural processing of placebo hyperhedonia and analgesia. "In order to compare the brain mechanisms of placebo hyperhedonia and analgesia, we assessed the effect of placebo treatment on subjective experiences within the same sensory modality – namely, touch, both pleasant and painful."
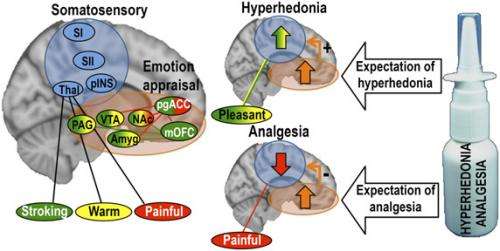
A key aspect of the study's analytic design was based on the researchers' knowledge that all dermal information is processed in the same neural pathways – specifically, the sensory thalamus, primary and secondary somatosensory areas, and the posterior insula. "As a result," Ellingsen points out, "we were able to perform two important measurements: we directly compared how expectation of improvement affected the processing of positive and negative somatosensory signals in these pathways, and investigated the effect of higher-level modulatory circuitry on sensory processing of pleasant or painful touch."
Another factor the scientists had to consider was that the use of subjective rating scales varies widely between individuals (as opposed to a single individual's typical consistency). As a result, these scales are significantly better at detecting changes between placebo and no-placebo within individuals rather than between one group who received placebo and another that received no placebo. "Consequently," Ellingsen explains, "such a design has superior statistical power – that is, a greater ability to detect a true effect."
Further, the potential benefit of a crossover design can be found when the order of treatment – specifically, placebo or no-placebo first – is considered, since it may potentially affect responses. "To control for this potential confounder," notes Ellingsen, "we used a crossover design, that is, half of the subjects got placebo in the first session, and the other half got placebo in the last session." However, he adds, in the analyses they performed, they found that the treatment order had no effect on either subjective placebo improvement or brain activation.
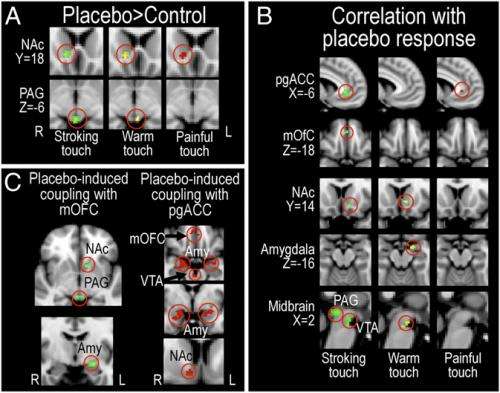
"To our knowledge," Ellingsen continues, "our study is the first to investigate placebo improvement of pleasurable feelings. By directly comparing this effect with the more well-known placebo analgesia effect, we were able to identify both the differences and a potential shared mechanism of these two types of improvement: People with stronger placebo-induced increases in functional coupling between ventromedial prefrontal cortex (vmPFC) and subcortical structures (PAG) reported greater placebo hyperhedonia and analgesia, and had greater analgesic decreases and hyperhedonic increases in somatosensory processing."
Ellingsen says that this finding suggests that endogenous improvement of positive and negative feelings are tightly coupled. "Interestingly, we saw that people with the greatest placebo hyperhedonia responses also had the greatest placebo analgesia responses. Overall, the results provide a piece of the puzzle of how positive expectations affect both positive and negative feelings."
Expanding on the team's findings, Ellingsen describes how the researchers first observed that placebo hyperhedonia was associated with increased activation of a number of cortical and subcortical areas important for placebo analgesia – namely, the ventromedial prefrontal cortex, accumbens, amygdala, and the midbrain structures periaqueductal grey and the ventral tegmental area. Not only was there increased activation in these areas after placebo administration compared to no-placebo, Ellingsen adds, but the amount of increase was positively correlated to the magnitude of the reported improvement: Those with largest placebo-induced hyperhedonia and analgesia had the highest placebo-induced activation in these areas. Moreover, those with largest placebo hyperhedonia and analgesia also had the strongest placebo-induced increase in functional connectivity within this circuitry, a measure of how much these areas communicate with each other. "Although our findings show similar patterns of activation between placebo hyperhedonia and analgesia, it's important to point out that they weren't identical. There are likely to be fine-grained differences between these processes within this circuitry that were not identified by this study."
Ellingsen stresses that an important mechanism in placebo analgesia – one that has been replicated several times – is the engagement of the opioid descending modulatory system, which consists of vmPFC, amygdala, and PAG. "When treated with a placebo that is expected to have analgesic effects," Ellingsen explains, "activation of this system suppresses nociceptive" (the neural processes of encoding and processing noxious or painful stimuli) "signaling both in the brain and – since the PAG has descending connections through the rostroventral medulla, RVM, to the spinal dorsal horn, where it can modulate incoming nociceptive signals – at the spinal cord level." Importantly, he notes, placebo analgesia and the activation of this system are reversed when the individual is given the opioid receptor antagonist naloxone, indicating that this mechanism is dependent on opioid signaling.
To ask whether this system is involved also in placebo improvement of pleasantness, we assessed the relationship between 1) the placebo-induced change in functional connectivity between the vmPFC and PAG, and 2) placebo-induced change in sensory processing. Strikingly, we found that the co-activation of vmPFC and PAG was related to opposite effects during placebo hyperhedonia and analgesia: During pain, those with strongest increases in functional coupling had the largest decreases in sensory processing, while during pleasant touch, those with strongest functional coupling had the largest sensory increases. We are now planning to investigate whether placebo hyperhedonia, like (most) placebo analgesia, depends on opioid signaling.
Moving forward, Ellingsen says, their study opens up several important questions for future studies:
- Does placebo hyperhedonia, similar to analgesia, rely on opioid or dopamine signaling?
- Could expectation of hyperhedonia alone have analgesic effects – and vice versa?
- Could including information about potential hyperhedonic effects actually boost treatment effects of analgesic drugs?
- What is the exact mechanism of the up-regulation of sensory processing in placebo hyperhedonia? Is it entirely central in its action, or could it involve descending facilitation of touch processing at the spinal cord level, which is a component in placebo analgesia4 and nocebo hyperalgesia5?
(A nocebo – the opposite of a placebo – is a harmless substance that creates detrimental effects in a patient who takes it. Likewise, the nocebo effect is the negative expectation-based reaction experienced by a patient who receives a nocebo.)
Regarding other areas of research that might benefit from their study, Ellingsen cites a growing recognition that health care systems need to be remodeled to target placebo mechanisms – and to do so by altering expectations, motivation, treatment context, and the therapist-patient relationship. "In most medical settings, however, the focus is to ease negative symptoms – to relieve pain, nausea, or discomfort – but to attain positive feelings, people have to seek elsewhere, despite our knowledge that positive experiences, like captivating music, pleasant odors, beautiful pictures, pleasant touch, and support from people we care about, can have potent analgesic effects."
If the tightly-coupling expectations of improvement in pleasurable and painful feelings suggested by their results interact in the clinical setting, Ellingsen believes it to be very likely that increasing the focus on positive appetitive effects of medical care (increased life quality, regained ability to enjoy pleasures, and the like) may have potent effects on the relief of negative symptoms. "In general," he concludes, "our findings shed some light on the complex relationship between positive feelings, negative feelings and expectation in the context of medical treatment. We believe our findings are relevant to the field of medical research in general, and promote widening the scope of medical research to improvement of positive experiences and pleasure."
More information: Placebo improves pleasure and pain through opposite modulation of sensory processing, PNAS Published online before print October 14, 2013, doi:10.1073/pnas.1305050110
Related:
1Relief as a Reward: Hedonic and Neural Responses to Safety from Pain, PLoS ONE 6(4): e17870. doi:10.1371/journal.pone.0017870
2Opponent appetitive-aversive neural processes underlie predictive learning of pain relief, Nature Neuroscience 8, 1234-1240 (2005),
3The importance of context: When relative relief renders pain
Pleasant, Pain 2013 Mar;154(3):402-10, doi:10.1016/j.pain.2012.11.018
4Direct Evidence for Spinal Cord Involvement in Placebo Analgesia, Science 16 October 2009: Vol. 326 no. 5951 p. 404, doi:10.1126/science.1180142
5Facilitation of Pain in the Human Spinal Cord by Nocebo Treatment, The Journal of Neuroscience, 21 August 2013, 33(34): 13784-13790; doi:10.1523/JNEUROSCI.2191-13.2013
6Placebo-induced changes in FMRI in the anticipation and experience of pain, Science, 303(5661): 1162-1167 (2004), doi:10.1126/science.1093065
7Placebo in emotional processing—induced expectations of anxiety relief activate a generalized modulatory network, Neuron, 46(6), 957-969 (2005), doi:10.1016/j.neuron.2005.05.023
© 2013 Medical Xpress. All rights reserved.