Scientists uncover how a cell's 'fuel gauge' promotes healthy development
Salk scientists have revealed how a cellular "fuel gauge" responsible for monitoring and managing cells' energy processes also has an unexpected role in development. This critical link could help researchers better understand cancer and diabetes pathways.
This cellular fuel gauge is a protein complex called AMPK that oversees energy input and output to keep the cell running smoothly. If AMPK were a car sensor, for instance, it would be able to instruct the vehicle when to get gas or lower the air conditioning to save energy. Similarly, if the cell's fuel supply—nutrients—is scarce, AMPK slows down cell growth and changes its metabolism. Previously, Salk Professor Reuben Shaw discovered that AMPK could halt tumors' revved-up metabolism, as well as restore normal function to the liver and other tissues in diabetics.
"Even though there's great interest in AMPK related to diabetes and cancer, frankly nothing was known about how this fuel gauge process changes in different cell populations during development," says Shaw, senior author of the work and holder of the William R. Brody Chair. Aside from giving new insight into stem cell therapies, the work, published in March 2016 in Genes & Development, could also help refine cancer treatments.
"To begin, we used CRISPR technology to edit out two important components of the AMPK pathway in embryonic stem cells," says Nathan Young, Salk research associate and first author of the paper. "At first we didn't see any difference, but things became interesting when we prompted the cells to differentiate."
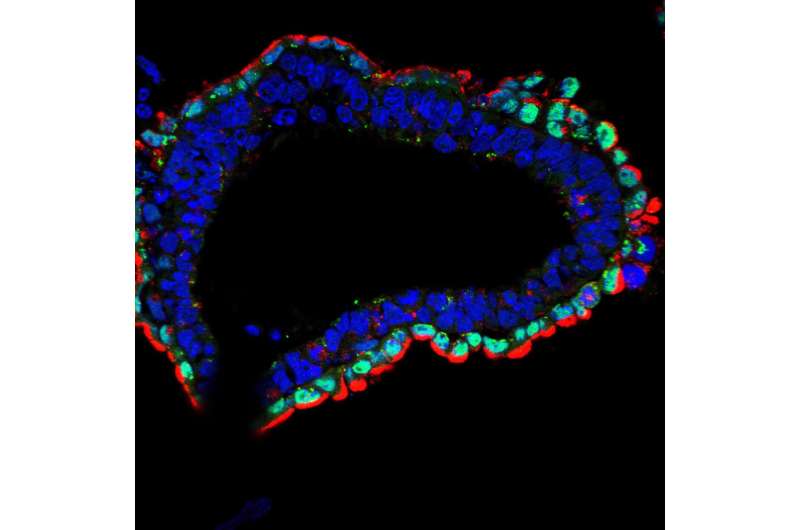
Normally, embryonic stem cells have the capacity to generate more specialized cells that belong to one of three broad groups termed germ layers—the endoderm, ectoderm and mesoderm—that can ultimately develop into all of the diverse cell types in an organism. However, the cells without a functioning AMPK pathway failed to efficiently make endoderm (the innermost layer in an organism) and instead made too much ectoderm (the layer that would turn into skin).
"These cells couldn't make the right choice," says Shaw. "This was the first inclination that this metabolic pathway is telling cells what kind of specialized tissues to become."
What was remarkable, according to the researchers, is when they looked closer at the gene expression patterns of the AMPK-deficient cells. They found that a large number of down-regulated genes related to one specific cellular structure: the lysosome. This critical self-contained organelle contains corrosive enzymes that degrade cellular material to reuse components—the garbage disposal and recycling center of the cell.
This loss of lysosomes, the researchers discovered, was due to the loss of a transcription factor called Tfeb, which turns on the expression of lysosomal genes in times of starvation. By simply reintroducing Tfeb into the dysfunctional cells, the team was able to restore normal development and differentiation.
"It was thought that lysosomes and AMPK were connected somehow, but no one had dreamed that you'd get no lysosomes if you don't have this fuel gauge," says Shaw. "Connecting the AMPK pathway to lysosomes begs the question of whether this pathway is part of anti-cancer pathways as well."
Currently, lysosome inhibitors are in dozens of clinical trials for breast, lung, pancreatic and brain cancers, even though the exact link between lysosomes and tumors are not understood. "We are decoding some of these underlying connections that might indicate when and how a cancer drugs might be useful," says Shaw. "This work may also help up make better, more specific ways of targeting lysosomes in cancer."
More information: Nathan P. Young et al. AMPK governs lineage specification through Tfeb-dependent regulation of lysosomes, Genes & Development (2016). DOI: 10.1101/gad.274142.115