Biomedical researchers break new ground in fight against multiple sclerosis

Researchers from the University of Maryland Fischell Department of Bioengineering and the University of Maryland School of Medicine report a new way to "turn off" the harmful immune attack that occurs during autoimmune diseases such as multiple sclerosis (MS), while keeping healthy functions of the immune system intact.
"Our lab is combining immunology and nanotechnology to reprogram how the immune system responds to self-cells in the brain that are mistakenly attacked during MS," said BIOE Assistant Professor Christopher Jewell, corresponding author on the new report. "The finding, conducted in cells and pre-clinical animal models of MS, could lead to new approaches for reversing paralysis in MS, or better therapies for other autoimmune diseases."
The group's findings were published September 13 in the journal Cell Reports. Collaborator Jonathan Bromberg, MD, PhD, a professor of surgery at the University of Maryland School of Medicine said, "The studies show it is possible to treatand cure inflammatory disease with a single dose of therapeutics loaded in biodegradable polymers targeted directly to lymph nodes – the tissues that coordinate immune function in the body."
In MS, the immune system incorrectly recognizes myelin that insulates and protects nerves fibers in the brain. Immune cells enter the brain and attack, leading to slow loss of motor function and other complications. Current therapies for MS work by decreasing the activity of the immune system; but, they do so in a broadly-suppressive way that often leaves patients vulnerable to infection. There are also no cures for MS, type 1 diabetes, and other autoimmune diseases.
"The goal of our work – and that of others in the field – is to expand cells that are both myelin-specific and regulatory in nature," said Lisa Tostanoski, first author on the paper. "The hope is that these cells can directly suppress inflammation without targeting healthy immune function."
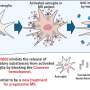
Jewell's team is working to reprogram the function of lymph nodes: instead of generating inflammatory cells that attack myelin, the lymph nodes are "instructed" to promote regulatory immune cells that control the attack against myelin. To carry out the "reprogramming," degradable polymer particles that incorporate regulatory signals are delivered to lymph nodes using a unique intra-lymph node injection technique. Once in thelymph nodes, these particles slowly release immune signals to promote regulatory immune cells that mature and migrate to the central nervous system to suppress the attack against myelin.
In order to test their strategy, the team is using two rodent models of MS. The results are promising thus far, demonstrating that a single particle treatment can permanently reverse paralysis. Importantly, these effects were found to be myelin-specific, and correlated with local changes in the function and types of cells in lymph nodes and the central nervous system.
"Moving forward, our team is working to show the therapeutic effects result from repair and remyelination in the brain," Jewell said. "That represents a goal that is a critical criterion to improve on human MS therapies."
To support this breakthrough research, the National Multiple Sclerosis Society has awarded the team more than $600,000 in research funding.
"This innovative research has the potential to open up a new, highly selective approach to treating multiple sclerosis," said Bruce F. Bebo, Ph.D., Executive Vice President of Research at the National Multiple Sclerosis Society. "We are pleased to have helped launch this work with early pilot and full research grants, and hope that the further research required to translate these results to people is equally successful."
"This kind of institutional collaboration can yield tremendous results," said UM SOM Dean E. Albert Reece, MD, PhD, MBA, who is also vice president for medical affairs at the University of Maryland and the John Z. and Akiko K. Bowers Distinguished Professor. "If we are to tackle the most critical and complex diseases, we must utilize multiple specialties across departments, schools and campuses to develop new treatments."
More information: Lisa H. Tostanoski et al. Reprogramming the Local Lymph Node Microenvironment Promotes Tolerance that Is Systemic and Antigen Specific, Cell Reports (2016). DOI: 10.1016/j.celrep.2016.08.033