A more detailed understanding of cell divisions giving rise to sperm and egg cells could lead to infertility treatments
Researchers have shown that a recently identified protein, called Speedy A, plays an essential role in the early stages of meiosis—a special type of cell division that produces sperm and egg cells.
In meiosis, a single cell divides twice, producing four cells, known as sperm or egg cells, which contain half the genetic information of the original cell. When a sperm fertilizes an egg, the resultant embryo contains a full set of chromosomes. In the early stages of meiosis, chromosomes residing in the nucleus undergo a process called recombination, which involves the exchange of genetic material that leads to genetic diversity.
"Recombination can only happen when the ends of the chromosomes, called telomeres, are attached to the nuclear envelope," explains Philipp Kaldis of the A*STAR Institute of Molecular and Cell Biology.
Kaldis, in collaboration with Kui Liu of Sweden's University of Gothenburg, and colleagues in China and the US, wanted to understand how chromosomal telomeres attach to the nuclear membrane or 'envelope', during meiosis.
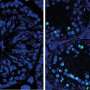
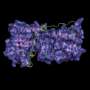
Using immunofluorescent staining of mouse spermatocytes, they found that a protein called Speedy A is localized to telomeres. Speedy A is a member of the Speedy/RINGO protein family, which activate cyclin-dependent kinase 2 (Cdk2), an important cell division-related protein which is also localized to telomeres, but whose role in meiosis is not fully understood.
The researchers then bred mice that were deficient in the gene for Speedy A and found that mice lacking Speedy A were infertile, similar to mice that were previously bred lacking Cdk2.
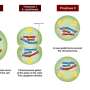
By comparing telomere–nuclear envelope attachment in mice with and without Speedy A, the team found that a specific portion of the Speedy A protein, called its RINGO domain, facilitated binding to Cdk2. Speedy A also bound to telomeres via its N terminus (the end that has a free amine group) and this, together with the RINGO domain, form Speedy A's 'telomere localization domain', which the researchers believe mediates the initial binding of chromosomal telomeres to the nuclear envelope.
Speedy A's other end, the C terminus (which has a free carboxyl group), is responsible for activating Cdk2 and is unlikely to affect telomere attachment to the nuclear membrane. Speedy A may also recruit Cdk2 to telomeres and later activate it together with other cyclins. Activated Cdk2 may then help regulate chromosome movements along the nuclear envelope.
"Our work is basic research, but you wonder whether a man with fertility defects may have defects associated with Cdk2 and Speedy A," says Kaldis. The team's "ultimate goal is to develop treatments for males with fertility issues," he says.
More information: Zhaowei Tu et al. Speedy A–Cdk2 binding mediates initial telomere–nuclear envelope attachment during meiotic prophase I independent of Cdk2 activation, Proceedings of the National Academy of Sciences (2017). DOI: 10.1073/pnas.1618465114