Scientists zero in on treatment for Charcot-Marie-Tooth disease

About 1 in 2,500 people have a degenerative nerve disease called Charcot-Marie-Tooth (CMT). The disease is typically diagnosed in children, who can lose their ability to walk and use their hands for fine motor skills. There is no cure—yet.
Scientists at The Scripps Research Institute (TSRI) have now shown a path to developing treatments for disease subtype CMT2D. As they report in the journal Nature Communications, it may be possible to reverse the disease by using a small molecule to restore normal protein function in the nervous system.
"This study provides guidance for developing therapeutics," says Xiang-Lei Yang, PhD, TSRI professor and senior author of the study.
Importantly, the study reveals how a better understanding of the fundamental causes of CMT can point researchers toward a cure for other subtypes.
Detective work reveals new role for mutant protein
Here's a puzzle: CMT2D is caused by mutations in a protein called GlyRS, which is expressed by cells throughout the body. Yet, the disease only damages the peripheral nervous system—the nerves in hands and feet.
Adding to the mystery, studies show that GlyRS primarily affects a process called protein synthesis, where genetic information is translated into proteins. Again, this process happens in all cells, so why would hands and feet be most affected?
"Our everyday research is like a detective role," says Zhongying Mo, PhD, senior research associate at TSRI and first author of the study.
The new study offers the answer: GlyRS has a role outside protein synthesis.
The researchers discovered that mutations in GlyRS trigger unusual interactions between GlyRS and a protein called HDAC6. Normally, HDAC6 would regulate a process called acetylation, which readies a protein called α-tubulin for its role in forming microtubules. Yang compares microtubules to a highway. Thanks to α-tubulin, signaling proteins and other important molecules can zip along, sending signals from your tiptoes to your brain.
But in CMT, the aberrant protein interactions with HDAC6 prevent proper α-tubulin acetylation, turning that highway into a dirt road. Nervous system signals can't run smoothly, and the longer the nerve, the rougher the road. Because our longest nerves reach our feet and hands, this finding explains why CMT2D is most severe in the peripheral nervous system—even though the mutant proteins are everywhere in the body.
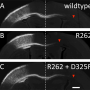
Further experiments in a mouse model of CMT2D showed that researchers could bring back proper nerve function by injecting the mice with a small molecule that blocks HDAC6 from interfering in α-tubulin acetylation. Although this particular small molecule would not be safe for humans to take, Yang and Mo believe a similar molecule may work as a future CMT2D therapy.
"It's exciting when you can accumulate all the evidence and point to a specific target," says Mo.
Targeting the root cause of CMT
Yang and Mo are excited to find this potential treatment target, but their ultimate goal is to treat the root cause of all types of CMT. To do this, they need to do more studies like this one, which reveal the fundamental pathology of the disease.
From patient to patient, different mutations can cause either mild or very severe symptoms. Some types of CMT are diagnosed in infancy, while others don't appear until adolescence. "That variability is striking," Yang says.
Now that the researchers know about this GlyRS interaction with HDAC6, they would like to investigate where else mutant proteins in CMT are causing problems. In fact, an earlier study from the Yang lab caught another problem made by the mutant proteins, which has something to do with affecting nerve maintenance signal. Yang hopes future studies can solve these mysteries and even show a way to target mutant GlyRS itself.
"Our understanding of the disease is ever-increasing," says Yang.
More information: Zhongying Mo et al, Aberrant GlyRS-HDAC6 interaction linked to axonal transport deficits in Charcot-Marie-Tooth neuropathy, Nature Communications (2018). DOI: 10.1038/s41467-018-03461-z