Inflammatory signals in heart muscle cells linked to atrial fibrillation

Interfering with inflammatory signals produced by heart muscle cells might someday provide novel therapeutic strategies for atrial fibrillation, according to an international team of researchers who have published their findings in the journal Circulation.
"Atrial fibrillation is the most common heart arrhythmia, and it is particularly observed in the elderly human population, which is growing worldwide," said corresponding author Dr. Na Li, assistant professor of medicine and of molecular physiology and biophysics at Baylor College of Medicine. "Atrial fibrillation can increase a person's risks for stroke and related heart problems."
Inflammation has long been implicated in many cardiovascular diseases including atrial fibrillation, but only as a bystander. This has changed as significant clinical evidence suggests that the inflammatory response, including inflammatory mediators called cytokines, are strongly associated with the progression of this disease. In this study, Li and her colleagues set out to determine whether inflammatory signaling could be playing a causative role in atrial fibrillation.
"We focused on an inflammatory signaling called NLRP3 inflammasome, which is typically associated with innate immune cells, like macrophages and monocytes," Li said. "Little is known, however, of the role this inflammasome plays in heart cells. We looked at the heart-specific inflammasone and asked what functions it plays in the heart and its possible link to atrial fibrillation."
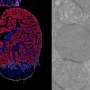
Li and her colleagues worked with both a mouse model of atrial fibrillation and with human heart tissue from patients with the condition. In the mouse model, the researchers genetically engineered mice to express a form of NLRP3 inflammasome that is constantly active only in heart cells, and then assessed whether these mice were more vulnerable to atrial arrhythmia.
"We found that mice with active NLRP3 inflammasome in their heart cells developed precursors of sustained atrial arrhythmia, such as spontaneous atrial contractions," Li said. "These spontaneous atrial contractions were suppressed by treating the mice with a specific NLRP3 inflammasome inhibitor or by specifically knocking down the NLRP3 inflammasome gene in heart cells. This indicates that in this model, activation of this inflammasome in heart cells alone is sufficient to promote events that have often been associated with the development of atrial fibrillation."
Looking to determine a human connection, the researchers analyzed human heart cells from patients with atrial arrhythmia and found increased levels of NLRP3 inflammasome activity, supporting their idea that this inflammasone may play a role in the human condition.
"It is well known that the function of the NLRP3 inflammasome is to prime immune cells for the release of cytokines that mediate an inflammatory response. Here we show for the first time that this inflammasome pathway also can mediate in a non-immune cell—a heart cell—other functions that are independent of producing cytokines," Li said. "To our surprise, we found that the activation of this inflammasome pathway in heart cells can affect many proteins that are involved in modulating the electrophysiology of cardiac cells. Enhancing this pathway ultimately leads to abnormal electrical patterns that are similar to those observed in atrial fibrillation in the mouse model."
The researchers propose that combining the results of previous clinical trials with their basic research findings can lead to novel therapies based on targeting the inflammasone pathway in heart cells.
Li and her colleagues are now looking into factors that may trigger activation of inflammasome signaling in heart cells.
"There is clinical evidence that modifying risk factors for atrial fibrillation—obesity, diabetes and sleep apnea, for instance—can lead to significant reduction of atrial fibrillation incidence," Li said. "We want to investigate whether these factors, which are also commonly associated with an inflammatory response, can in addition promote atrial fibrillation through the inflammasome pathway in heart cells."
More information: Circulation (2018). DOI: 10.1161/CIRCULATIONAHA.118.035202