Researchers breathe new life into COPD research using mouse models
The airways and lungs are affected by a range of diseases, but while some have been well characterized and treated, others, such as chronic obstructive pulmonary disorder (COPD), are still somewhat of an enigma. This condition is known to involve a variety of progressively deteriorating symptoms such as inflammation of the lungs and narrowing of the airways, but the causes of these at the cellular and molecular levels have largely remained obscure.
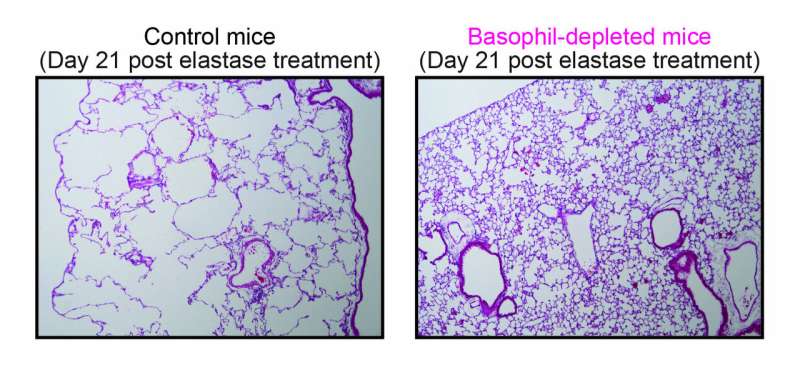
In a landmark study recently reported in the journal Proceedings of the National Academy of Sciences, a research team from Tokyo Medical and Dental University (TMDU) used a variety of mouse models to explain the developmental process behind COPD. The team showed that the immune cells basophils, previously believed to be mainly involved in fighting parasitic infections and inducing allergic responses, also induce the destruction of alveolar walls (emphysema).
Earlier studies of COPD focused on mice as a suitable model for easily dissecting the mechanisms behind this disease, but encountered a range of difficulties in mimicking the features in humans. First, mice were exposed to smoke, given the association between smoking and COPD, but this failed to mirror the progressiveness of the disease in humans even after they quit smoking. This was superseded by the approach of administering enzymes called proteases directly into the airway. Proteases break up proteins, producing more human COPD-like features.
"Here, we induced emphysema in model mice using a protease called elastase," lead author Sho Shibata says, "We then worked backwards one step at a time through the series of cellular and molecular events causing this symptom to identify what started this cascade, using mice with knockout of each component of this cascade."
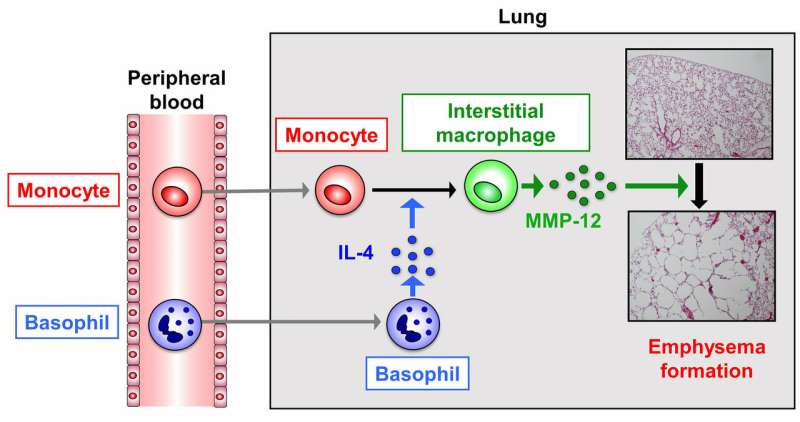
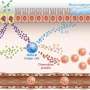
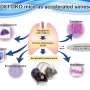
The team started by revealing that the lung tissue destruction was caused by a molecule called MMP-12, which they found was released in excess from immune cells called interstitial macrophages when elastase was administered. They then found that these interstitial macrophages arose when the precursor immune cells called monocytes were induced by an immunity-regulating molecule called interleukin-4. Looking backwards another step in this chain, the team showed that interleukin-4 was released by basophil immune cells.
Control mice (left panel) or basophil-depleted mice (right panel) were intranasally treated once with porcine pancreatic elastase to elicit emphysema.
"Our results are surprising because basophils have not previously been identified to be active in this kind of situation," corresponding author Hajime Karasuyama says. "It may be that basophils were previously overlooked because they only make up 1 percent of the white blood cells in the lungs."
Basophil-derived IL-4/monocyte-derived IM/MMP-12 axis plays a crucial role in emphysema formation The team hopes that their discovery will lead to therapies targeting basophils or the interstitial macrophages that they create to slow down the progression of emphysema.
More information: Sho Shibata et al, Basophils trigger emphysema development in a murine model of COPD through IL-4–mediated generation of MMP-12–producing macrophages, Proceedings of the National Academy of Sciences (2018). DOI: 10.1073/pnas.1813927115