Radiation-free stem cell transplants, gene therapy may be within reach
Researchers at Stanford and the University of Tokyo may have cracked the code to doing stem cell transplants and gene therapy without radiation and chemotherapy.
For decades, researchers have been stymied in their attempts to grow large numbers of hematopoietic stem cells in the laboratory. These rare bone marrow cells are solely responsible for generating all the cells of the blood and immune system. Difficulties in growing the cells have seriously hampered many research efforts, including those aimed at making stem cell transplantation or gene therapy in patients with certain cancers or blood disorders easier and safer.
Now, researchers at the Stanford University School of Medicine and the University of Tokyo have cracked the code. By tinkering with the components of the nutritive broth in which the cells are grown, the specialized molecules used to support their growth and the physical conditions under which the cells are cultivated, the researchers have shown for the first time that it's possible to coax hematopoietic stem cells from mice to renew themselves hundreds or even thousands of times within a period of just 28 days.
"This has been one of my life goals as a stem cell researcher," said Hiromitsu Nakauchi, MD, Ph.D., professor of genetics at Stanford. "For 50 years, researchers from laboratories around the world have been seeking ways to grow these cells to large numbers. Now we've identified a set of conditions that allows these cells to expand in number as much as 900-fold in just one month. We believe this approach could transform how hematopoietic stem cell transplants and gene therapy are performed in humans."
In particular, the researchers have shown it is possible to successfully transplant large numbers of the cells into mice without first eliminating the recipients' own stem cell population. If the technique also works in humans, it could save thousands of patients with blood or immune disorders from a grueling regimen of radiation or chemotherapy prior to transplant. It could also allow clinicians to use a patient's own genetically corrected stem cells for gene therapy.
The study was published online May 29 in Nature. Nakauchi shares senior authorship of the study with Satoshi Yamazaki, Ph.D., an associate professor of stem cell biology at the University of Tokyo. Postdoctoral scholar Adam Wilkinson, Ph.D., of Stanford and senior research assistant Reiko Ishida of the University of Tokyo are the lead authors.
Hematopoietic stem cells are rare cells found in the bone marrow. Like other stem cells, they can either divide to make more hematopoietic stem cells—a process called self-renewal—or generate the precursors of all the different types of blood and immune cells in the body—a process called differentiation.
It's long been known that people with immune or blood disorders such as sickle cell anemia or leukemia can be cured with a transplant of healthy hematopoietic stem cells. But in order for the treatment to work, the recipient's own hematopoietic stem cells must be killed to eliminate the disease and make space for the healthy cells to settle in the bone marrow.
This elimination step, also called "conditioning," is accomplished with either chemotherapy or radiation, or a combination of the two. The conditioning, however, can cause life-threatening side effects.
This is particularly true in pediatric patients, who can suffer side effects such as growth retardation, infertility and secondary cancer in later life. Very sick or elderly patients often can't receive transplants because they are unable to tolerate the conditioning treatment.
"A Holy Grail in the stem cell field'
But for some time, researchers have wondered whether transplanting large numbers of donor hematopoietic stem cells could circumvent the need to remove the existing cells. Perhaps, they reasoned, swamping the recipient's bone marrow with a tide of healthy donor cells would allow the newcomers to muscle their way in and set up shop making healthy blood and immune cells.
It's been difficult to test this theory, however, because hematopoietic stem cells are hard to isolate in large numbers. And although it's been possible for years to grow human hematopoietic stem cells in the laboratory, the cells never self-renew robustly and instead often abandon their stem cell fate to differentiate into precursor cells. As a result, it's been difficult to study their biology in depth or to generate enough cells to attempt large-scale transplants.
"Expansion of hematopoietic stem cells has been a Holy Grail in the stem cell field," Nakauchi said. "When something does not work, people tend to think that something is missing. But we decided to try the opposite approach by eliminating all the impurities in the conventional culture system, while also optimizing other aspects that encourage self-renewal of the hematopoietic stem cells."
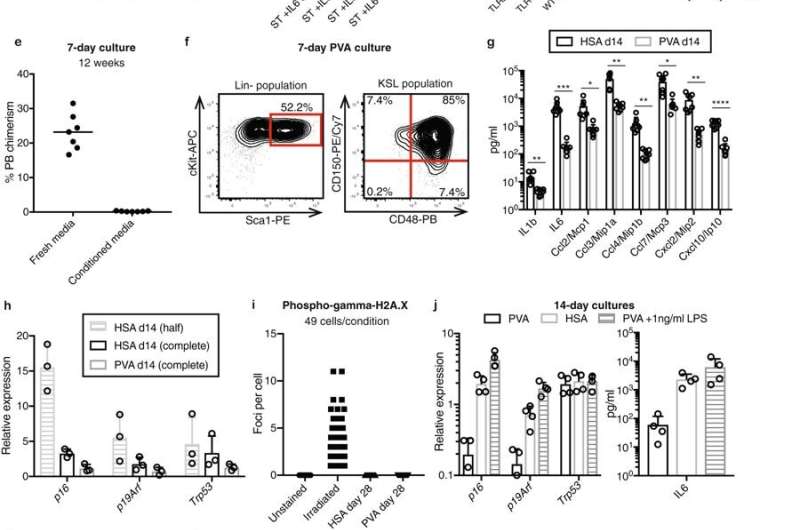
The researchers also replaced a potentially contaminated blood protein called serum albumin with polyvinyl alcohol, a water-soluble synthetic chemical that is frequently used in biomedical research.
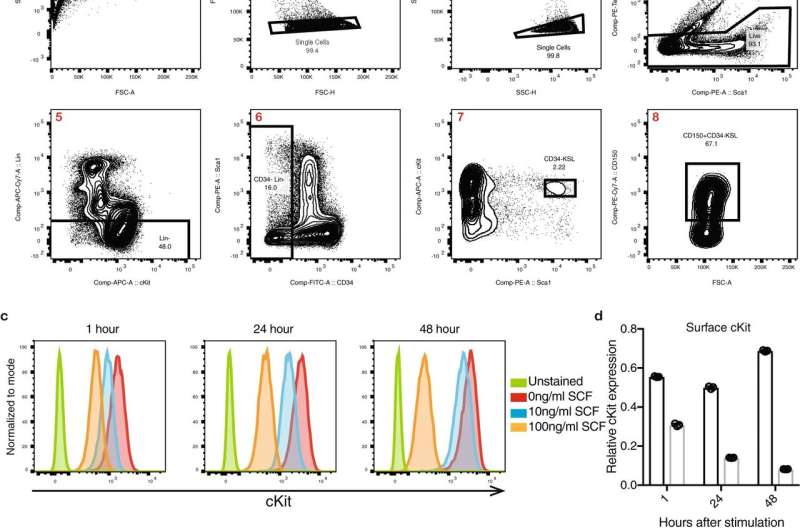
These modifications to how the cells were grown allowed the researchers to generate enough hematopoietic stem cells for transplant from just 50 starting cells. Although some of the cells did differentiate in culture, many more than normal maintained their stem cell identity throughout the duration of the culture.
"These original 50 cells increased in number about 8,000-fold over 28 days," Nakauchi said. "Of these, about one of every 35 cells remained a functional hematopoietic stem cell."
Planning to test human cells
The researchers estimated that the hematopoietic stem cells in the original sample increased in numbers by between 200- to 900-fold—an unprecedented level of expansion. When they transplanted the newly grown cells into mice that had not undergone a conditioning regimen, the animals developed blood and immune cells derived from both the donors' hematopoietic stem cells and their own, demonstrating that the donor cells had engrafted and remained functional.
"We also found that, during the culture, we can use CRISPR technology to correct any genetic defects in the original hematopoietic cells," Nakauchi said. "These gene-corrected cells can then be expanded for transplantation. This should allow us to use a patient's own cells as gene therapy."
Nakauchi and his collaborators at Stanford are now testing this approach in mice.
More information: Adam C. Wilkinson et al. Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation, Nature (2019). DOI: 10.1038/s41586-019-1244-x