Muscle stem cell technology a prelude to new muscular dystrophy therapeutics
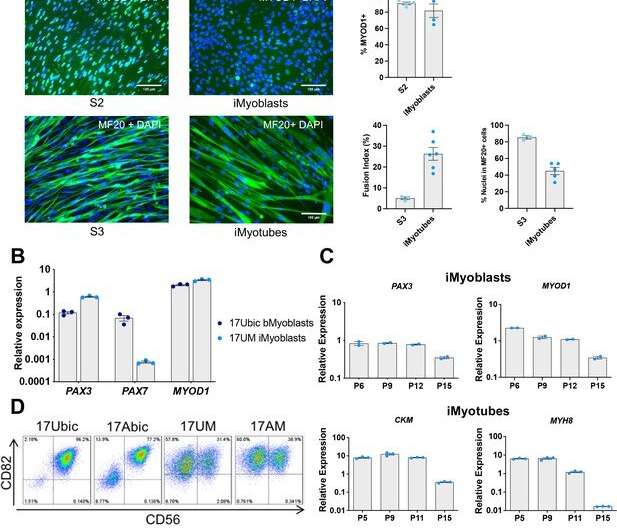
Scientists at UMass Chan Medical School have developed a technology to isolate human skeletal muscle stem cells, or progenitor cells, from induced pluripotent stem cells (iPSCs). Christened iMyoblasts in an eLife paper by corresponding investigator Charles P Emerson Jr., Ph.D., these patient-derived muscle stem cells enable researchers to pursue laboratory investigations into the earliest impacts of disease-causing mutations on muscle formation and function.
Patient-derived stem cells, such as iMyoblasts, are a core foundation for preclinical laboratory models for many known human muscular dystrophies. iMyoblast technology has the power to advance gene therapy for human muscular dystrophies—using strategies including RNA silencing, DNA editing and stem cell therapy—for clinical applications.
"This is a critical step for the development of gene editing treatments," said Dr. Emerson, professor of neurology. "Laboratory models of human muscular dystrophy are required to develop these therapeutics before clinical use in patients.
iPSCs can be readily produced in tissue culture by reprogramming of patient's somatic cells, including skin and muscle biopsy cells, said Emerson.
"Using molecules known to direct muscle cell maturation during development, we can create muscle progenitor cells that can both differentiate into skeletal muscle and reproduce themselves to regenerate or repair muscle," he said. "This becomes an important tool in our toolbox to investigate and develop therapeutics for muscular dystrophies."
There are more than 40 known muscular dystrophies caused by genetic mutations that affect muscle function. These disorders have variable ages of onset and clinical severity, but most often result in severe physical disabilities and premature death. Over what can be decades, patients with muscular dystrophy experience progressive muscle weakness and decreased mobility, making everyday tasks difficult and often impossible to perform even during early disease. Overall, there are fewer than 200,000 cases of muscular dystrophy diagnosed each year in the United States, but this prolonged disease progression places a significant long-term burden on patients and their families and the health care system.
The primary benefit of iMyoblasts is their ability to regenerate, or multiply, to create more progenitor cells in addition to differentiating into mature muscle cells. More than 25 years ago, scientists unraveled the biology behind how embryos make mature muscle. Another leap forward came about 10 years ago when methods were developed to produce differentiated muscle from patient iPCSs. But these earlier technologies were limited in their ability to make muscle stem cells that can both differentiate and regenerate muscles. Their utility in the laboratory and clinic, where organisms need to respond to injury and age, were hampered by these constraints.
In contrast, skin cells taken from a patient with any form of muscular dystrophy can be turned into iMyoblast progenitor cells. When iMyoblasts are then engrafted into animal models, they give rise to adult human muscle cells with the muscular-dystrophy-causing mutation. These disease models are key to developing new therapeutics with the potential to treat muscular dystrophies.
"Developing models of all these different muscular dystrophy mutations is difficult," said Emerson. "Not only are there more than 40 unique forms of muscular dystrophy and a multitude of mutations, but obtaining muscle cells from a patient normally requires an invasive muscle biopsy. And what you do get from the biopsy are often damaged stem cells. With the iMyoblasts, all we need are a few patient skin cells and we can have animal and cell culture models to study."
Using iMyoblasts, Emerson and his group were able to develop animal models for four distinct forms of muscular dystrophy: facioscapulohumeral muscular dystrophy, limb-girdle muscular dystrophy types R7 and R9, and Walker-Warburg syndrome. These models successfully replicated the molecular disease pathologies of the disorders and were responsive to small molecule and gene editing therapeutics.
The hope is that eventually iMyoblasts can be used in combination with gene editing and stem cell therapeutics to alleviate or cure a broad range of muscular dystrophies.
"The long-term goal is that we can develop gene editing technology to fix the disease-causing mutations in the iMyoblasts, which can then be transplanted back into patients where they go on to build healthy muscle tissues. And because the iMyoblasts are self-renewing, the hope is that these cells will be able to respond to injury in the adult human environment and continue generating new, healthy muscle cells over an extended period," said Emerson.
More information: Dongsheng Guo et al, iMyoblasts for ex vivo and in vivo investigations of human myogenesis and disease modeling, eLife (2022). DOI: 10.7554/eLife.70341