This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study shows effect of sex and prior exposure on vaccine immune responses
Scientists have described the differing responses that occur in response to a herpes simplex virus (HSV) 2 vaccine, based on a patient's sex and their prior exposure to the virus.
The results, published today in eLife, suggest that women have a larger magnitude of early immune responses compared to men, as measured by their interferon (IFN) signatures—a group of signaling proteins that are released by host cells in response to a virus. IFN signatures were found to be most pronounced in women who had not previously been exposed to HSV.
People's immune responses have previously been shown to differ based on sex. Females typically show a larger antibody response than males. Antibodies are proteins made by plasma cells which fight infection and are produced in response to an antigen—the marker of a virus that is recognized by the immune system. Prior exposure to an infection has also been suggested to influence the immune response, but studies have not looked at the combined effect of the two traits on vaccination responses in humans.
"We wanted to investigate the effects of the interaction between sex and prior exposure on people's responses to a vaccine," explains co-senior author John Tsang, professor of immunobiology and biomedical engineering at Yale, "To do this, we leveraged a unique cohort that received an experimental, multi-dose HSV2 vaccine, and were able to compare the volunteers' responses to the vaccine based on their HSV serostatus prior to vaccination," adds co-first author Foo Cheung, Staff Scientist at the Center for Human Immunology, National Institutes of Health, Bethesda, US, which Tsang co-directed when this research was performed.
Serostatus refers to the presence or absence of antibodies related to a particular virus, measured by a blood test. HSV is categorized into two types; HSV1 and HSV2. HSV1 is more commonly associated with symptoms such as cold sores, and HSV2 is more commonly associated with genital herpes.
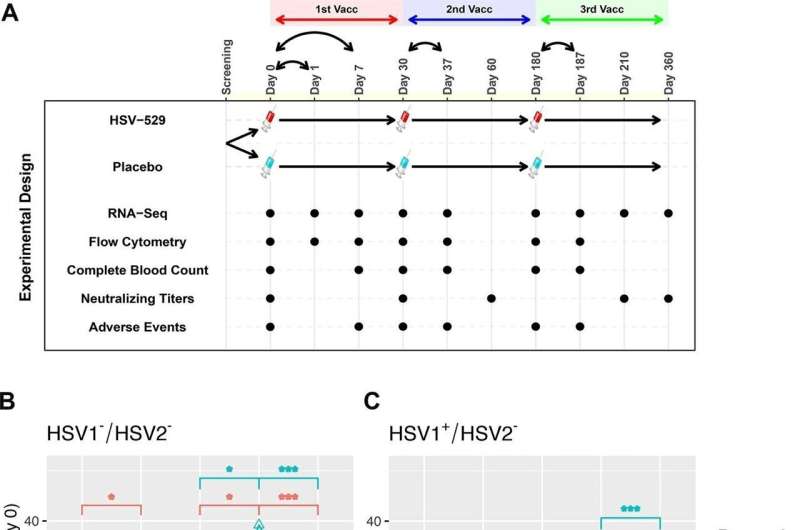
A total of 60 volunteers took part in the study, including an equal number of men and women, who were then split into three groups of 20. The first group included participants who were seronegative for both HSV1 and HSV2. The second group was seropositive for HSV1 and seronegative for HSV2, and the third group was seropositive for HSV2 and was HSV1 seropositive or seronegative. In each group, 15 participants were given three separate vaccine doses and five were given a placebo dose.
Cheung and colleagues used blood transcriptomics to observe which genes were changed after vaccination, and cell population profiling to determine the abundance of cell types present. They focused on the responses that occurred in participants one day after the first dose, as this timeframe reflects the early inflammatory processes that drive ensuing immune responses; these early responses on day one also exhibit the most amount of differences compared to a baseline sample just prior to vaccination.
The team measured and compared IFN transcriptional signatures between the groups. They discovered that IFN responses in seronegative participants skewed towards a type I IFN (IFN-ɑ), whereas seropositive participants skewed towards a type II IFN (IFN-?). The fact that IFN-? was more prominent in those previously exposed to HSV supports previous studies that have suggested IFN-? inhibits HSV reactivation.
The researchers also found that the magnitude of early transcriptional responses was largest in women who had not been exposed to HSV (otherwise known as HSV-naïve), and type I IFN signatures were particularly pronounced in this group. Within this group individuals that mounted more robust IFN responses demonstrated lower levels of vaccine-induced antibodies, suggesting that a strong, early antiviral response reduced the uptake of the virus vaccine. This is consistent with the established role of IFN in hindering HSV infection and indicates that the effects of IFN responses on vaccine effectiveness may be dependent on factors such as the person's sex.
The team calls for further study in this area to determine if the IFN responses seen in the HSV-naïve women are replicated in the responses of other infection-naïve people to different types of vaccine.
"Our findings add to and integrate the substantial effects seen for prior exposure and sex in innate immune responses," concludes co-senior author Jeffrey Cohen, Chief of the Laboratory of Infectious Diseases, Medical Virology Section, National Institutes of Health. "Further understanding of how prior exposure and sex interact to affect immune responses and shape subsequent adaptive immunity is crucial for the development of more effective vaccines."
More information: Foo Cheung et al, Sex and prior exposure jointly shape innate immune responses to a live herpesvirus vaccine, eLife (2023). DOI: 10.7554/eLife.80652