This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New study suggests a promising therapeutic target for sepsis
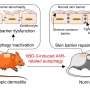
Sepsis, one of the most acute and serious disease complications in the intensive care unit, is caused by various infections and results in life-threatening organ dysfunction. The intestinal barrier plays a vital role in the process of sepsis, and its disruption exacerbates sepsis.
A new study in The American Journal of Pathology has found that promoting autophagy, the process by which cells break down and destroy damaged or abnormal proteins, with rapamycin, an immunosuppressant, reduced intestinal epithelial cell death and restored intestinal barrier function during sepsis.
The study also suggests that the interplay of mammalian target of rapamycin (mTOR), a negative regulator of autophagy, and polo-like kinase 1 (PLK1) is crucial in sepsis-induced barrier dysfunction and may provide novel insights for treatment of sepsis.
"Despite the increased understanding of sepsis pathophysiology and the application of advanced clinical treatments, sepsis remains a major cause of health loss worldwide with a high health-related burden," said lead investigator Wei-Hua Lu.
"Because the function of mTOR and PLK1 in sepsis remains unclear, further investigation is warranted."
In the study, mice were subjected to cecal ligation and puncture (CLP), a perforation of the cecum allowing the release of fecal material into the peritoneal cavity, which established a sepsis model in vivo.
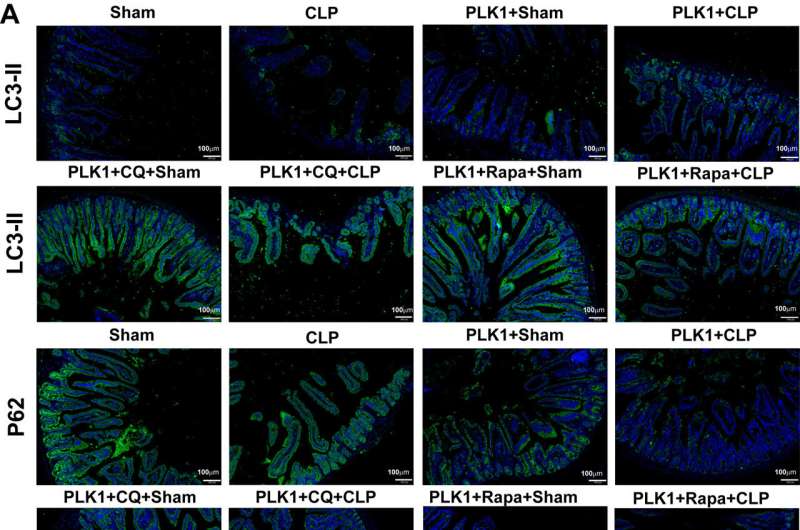
Compared to mice in sham group, the CLP mice had severe intestinal mucosal injury and increased intestinal mucosal permeability. Under rapamycin treatment, activation of autophagy inhibited enterocyte apoptosis and restored the disrupted intestinal barrier, suggesting that autophagy plays a protective role in sepsis-induced intestinal barrier dysfunction.
To determine whether the protective role of PLK relies on autophagy, mice modified with the PLK1 gene (CAG-PLK1 mice) underwent CLP. Activation of autophagy was observed, and apoptosis was alleviated. However, these ameliorative phenomena deteriorated in mice treated with chloroquine, an inhibitor of autophagy, compared with mice treated with rapamycin. These results indicate that PLK1 protects against sepsis-induced barrier dysfunction by promoting intestinal autophagy.
To further explore whether PLK1 promotes autophagy via the mTOR pathway in intestinal epithelial cells, the investigators observed the physical interaction between PLK1 and mTOR in an in vitro model of human colonic epithelial cells. They found that PLK1 also promotes cell autophagy and improves autophagy and high permeability. Moreover, PLK1 physically interacted with mTOR and participated in reciprocal regulatory crosstalk in intestinal cells during sepsis.
"The reciprocal regulation of the PLK1-mTOR axis is crucial in sepsis-induced intestinal barrier dysfunction," Dr. Lu said. "These findings indicate that the PLK1-mTOR axis may be a promising therapeutic target for treatment of sepsis."
More information: Ying-Ya Cao et al, The Polo-Like Kinase 1–Mammalian Target of Rapamycin Axis Regulates Autophagy to Prevent Intestinal Barrier Dysfunction During Sepsis, The American Journal of Pathology (2022). DOI: 10.1016/j.ajpath.2022.11.008