This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Statistics framework to remove bias from debate about how well mouse models mimic human disease
Mice and other animals have been key to some of the biggest medical breakthroughs in human history. But animals aren't always good models of human disease, leading to failed experiments and controversy over their usefulness.
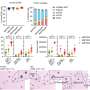
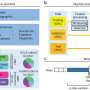
A team of biostatisticians led by University of Pittsburgh School of Public Health scientists announced today in PNAS that they've developed a framework to determine how much congruence and discordance laboratory animals have with specific human diseases. The tool removes potential bias from scientific interpretation of how translational animal data is for human conditions.
"There have been decades of debate about whether animal models mimic humans well and whether they are useful for translational or clinical research," said senior author George Tseng, Sc.D., professor and vice chair for research in Pitt Public Health's Department of Biostatistics. "Our framework is the first to provide quantitative methods and bioinformatic workflow to properly address that debate."
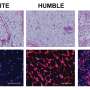
Tseng and his team tackled the topic after two papers published in PNAS—one in 2013 and one in 2014—that used the same datasets presented contradictory conclusions on the usefulness of mice as models of human diseases that involve inflammation, such as sepsis and burns.
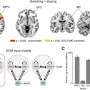
The team reanalyzed the datasets in the contradictory PNAS papers with their Congruence Analysis for Model Organisms (CAMO) framework. It found that for the six human inflammatory disorders studied, two were well mimicked by mice; two were not, and two did not have enough data to draw conclusions. Tseng's team determined that the previous studies reached different endpoints because the scientific teams—one mostly composed of laboratory-based scientists and the other primarily of clinicians—had used different thresholds, or cut-off points, for their analyses.
"The conclusion drawn by our unbiased, threshold-free framework is much more realistic," Tseng said. "In the end, you cannot say that the mouse model is totally useless or totally perfect. A mouse model can mimic some biological mechanisms well but others poorly. The issue is whether it mimics the mechanism of interest, such as the drug target. And it even revealed that the data aren't perfect in some situations—if you have limited information, you can't draw a conclusion."
The team is extending their research into cancer to examine which cell-cultured models are good mimics for tumors and psychiatric disorders to learn, for example, whether mice mimic the human circadian rhythm.
"We anticipate CAMO becoming an essential part of preclinical studies to solve all manner of human diseases," Tseng said.
More information: Wei Zong et al, Transcriptomic congruence analysis for evaluating model organisms, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2202584120