This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Long-range neuronal connections drive glioblastoma progression, new study reveals
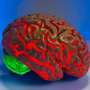
Glioblastoma (GBM) is the most aggressive and lethal form of brain tumor. Despite treatment, GBM recurrence is inevitable and tends to occur outside surgical margins or in locations remote to the primary tumor, highlighting the central role played by tumor infiltration in this malicious disease.
Little is known about the underlying molecular mechanisms driving GBM infiltration, but in a new study published in the journal Nature, researchers at Baylor College of Medicine working with animal models reveal a novel process by which neurons in locations remote to the primary tumor provoke expression of genes from glioblastoma that subsequently drive tumor infiltration.
"Previous studies have shown associations between the presence of GBM and increased neuronal activity in surrounding brain regions, which can promote tumor progression," said first author Dr. Emmet Huang-Hobbs in Dr. Benjamin Deneen's lab.
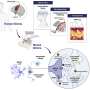
To study how neurons stimulate GBM infiltration, the researchers first determined which neuronal populations promoted glioma intrusion. They hypothesized that callosal projection neurons (CPNs) localized in the cortical hemisphere contralateral to the primary tumor contributed to this phenomenon. CPNs extend across the brain along the corpus callosum, a strip of white matter that connects the left and right cerebral hemispheres.
"Severing the corpus callosum eliminated the neuronal activity-dependent acceleration of GBM infiltration that was observed with the intact control, supporting that an intact corpus callosum is necessary to promote glioma progression and implicating CPNs' long-range projections in remotely driving GBM infiltration," Huang said.
"The findings suggest that GBMs receive neuronal inputs from a host of brain regions, implying that exposure to a diverse range of neuroactive compounds can potentially influence tumor growth. It's now clear that tumor-neuron interactions are more widespread than previously thought," said Deneen, professor and Dr. Russell J. and Marian K. Blattner Chair in the Department of Neurosurgery, director of the Center for Cancer Neuroscience and a member of the Dan L Duncan Comprehensive Cancer Center at Baylor. He also is the corresponding author of the work.
"In collaboration with the labs of Baylor researchers Dr. Jeffrey L. Noebels and Dr. Ganesh Rao, we found evidence suggesting that GBM and CPNs have a two-way conversation," Huang said. "CPNs promote tumor infiltration, and the tumor affects neuronal connections or synapses. The tumor remodels local neuronal synapses and makes direct synaptic connections, raising the possibility that it alters brain circuit activity in these regions that are distant from the primary tumor."
Further analyses showed mechanistic details underlying these observations. The researchers found that the infiltrating tumor population is enriched for axon guidance genes, including SEMA4F, which they identified as an essential factor for glioma progression and neuronal activity-dependent infiltration. Interestingly, SEMA4F also promotes neuronal hyperactivity.
"Taken all together, we propose a model in which neurons prompt the expression of genes from glioma tumors that subsequently drive infiltration and their own synaptic activity," Huang said. "A better understanding of the two-way conversation between GBM and CPNs is an important step toward improved brain tumor treatments."
More information: Benjamin Deneen, Remote neuronal activity drives glioma progression via Sema4f, Nature (2023). DOI: 10.1038/s41586-023-06267-2. www.nature.com/articles/s41586-023-06267-2