This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Low oxygen levels restore balance and coordination in a mouse model of a movement disorder

Friedreich's ataxia is a rare, inherited disease that causes progressive nervous system damage, impairing balance and coordination and leaving patients unable to walk by early adulthood.
Now, researchers at the Broad Institute of MIT and Harvard have found that treatment with continuous hypoxia—low-oxygen conditions comparable to levels at a Mount Everest base camp—restores balance and coordination in a mouse model of Friedreich's ataxia. With further development, the findings could inform a potential treatment that lowers oxygen levels in certain tissues in humans to reverse advanced disease. The study was published recently in Human Molecular Genetics.
"If we accept that disease stems from the interaction of genes and environment, then why not manipulate the environment?" said Vamsi Mootha, senior author of the study, an institute member at the Broad, professor of systems biology and medicine at Harvard Medical School, and professor of molecular biology at Massachusetts General Hospital.
"Through a series of papers we have reported a strong interaction between genetic pathways related to energy metabolism and environmental oxygen. By attacking disease from the environment side of things, we think our quest for a hypoxia-in-a-pill approach could impact a number of genetically diverse diseases."
Mootha's lab has been studying hypoxia as a potential treatment for rare neurological diseases since 2016. Previous work from his lab suggested that hypoxia could prevent ataxia—impaired balance and coordination—in Friedreich's ataxia mice, and the new findings now show that the approach can even reverse it in animals with more advanced disease.
The latest study also adds to a growing body of evidence that hypoxia could treat a broad range of conditions. Last month, for instance, Mootha's team showed that a mouse model of an accelerated aging disease lived 50% longer and showed slower neurological decline in low-oxygen conditions. The work appeared in PLOS Biology.
"Oxygen restriction is an exciting experimental modality, and it's now been shown to have pretty potent beneficial effects across multiple models of aging and disease," said Robert Rogers, first author on the PLOS study, postdoctoral fellow in Mootha's lab, and an instructor in the Division of Pulmonary and Critical Care Medicine at Massachusetts General Hospital. "It deserves a lot of further exploration to understand its mechanism so that we can recapitulate it in a form that's easier to deliver, like a pill."
Treatment comparison
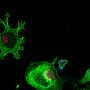
Friedreich's ataxia is caused by loss of a mitochondrial protein called frataxin, which helps make molecular complexes called iron-sulfur clusters required for a number of cellular pathways, including those involved in energy metabolism. Patients with Friedreich's ataxia have fewer of these clusters than people without the disease, resulting in impaired energy production in the affected cells.
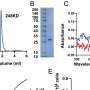
In 2019, Mootha's team found that continuously exposing young mice with Friedreich's ataxia to 11% oxygen, as opposed to 21% at sea level, early in the disease prevented ataxia. They also found that hypoxia restored iron-sulfur cluster levels in cells. However, most patients are diagnosed later in the disease, after symptoms develop, so the researchers wanted to test whether hypoxia could reverse—rather than simply prevent—advanced disease. And because extreme hypoxia can be harmful, the scientists knew they'd have to keep optimizing the treatment.
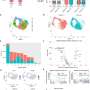
To look for other possible regimens, Mootha's team, in their latest study, tested seven different hypoxia treatments in the same mouse model of Friedreich's ataxia and included animals in later stages of the disease. A few treatments stood out.
Intermittent hypoxia—16 hours of 11% oxygen and 8 hours of 21% oxygen—was harmful, and mice developed cardiac stress and died more quickly than those without treatment. The group hypothesized that the animals' red blood cell levels increased due to hypoxia, and then when exposed to normal oxygen levels, created a temporary but toxic flood of oxygen. They supported this hypothesis by using a drug that pharmacologically blocks this response.
The team reported that treatment with mild hypoxia—17% oxygen, equivalent to the levels in Denver—was moderately beneficial, and mice developed ataxia later than those without treatment.
But the researchers were excited when they found that continuous hypoxia at 11% oxygen reversed neurological symptoms and ataxia, even at advanced disease.
"We've shown how amenable the nervous system is to recovering from damage," said Tslil Ast, a co-first author on the study along with Hong Wang, a research scientist in Mootha's lab. Ast was a postdoctoral fellow in Mootha's lab when the study began and is currently a team leader at the Weizmann Institute of Science in Israel. "Even with profound ataxia, these mice can still, under the correct therapeutic regimen, reverse course."
The treatment did not improve the disease's cardiovascular symptoms, suggesting that more work, potentially towards a combination therapy, is needed to transform these insights into a curative treatment.
In the PLOS study from last month, Mootha's group demonstrated that the same treatment—continuous exposure to 11% oxygen—increased lifespan by 50% in mice with mutations in the Ercc1 gene that lead to accelerated aging and early death in the animals.
In the future, the researchers aim to continue to uncover the full mechanism by which hypoxia alleviates ataxia, which could inform hypoxia-inspired therapies such as a pill that could decrease oxygen or blunt the body's ability to deliver oxygen to tissues.
In the meantime, Ast and Mootha say that hypoxia can be deadly if not done in a controlled clinical setting. For example, in the current work they noted that not only were intermittent regimens not helpful, but even harmful, underscoring the need for and value of continued pre-clinical testing before moving into humans.
More information: Tslil Ast et al, Continuous, but not intermittent, regimens of hypoxia prevent and reverse ataxia in a murine model of Friedreich's ataxia, Human Molecular Genetics (2023). DOI: 10.1093/hmg/ddad091