July 31, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study identifies ligand-receptor pairs driving the development of astrocytes
Astrocytes are a type of glial cell in the central nervous system that clear excess neurotransmitters, promote the formation of synapses (i.e., connections between neurons), and perform other functions. The development of astrocytes, like that of other glial cells, is partly supported by extrinsic ligands. Ligands are molecules that bind to a receiving protein, known as a receptor, eliciting various cellular responses.
Researchers at Emory University School of Medicine recently set out to identify ligand-receptor pairs that support the genesis and development of astrocytes in the human brain using computational techniques. Their paper, published in Nature Neuroscience, highlights the potential of analyzing large amounts of available data to identify new neuroscientific hypotheses.
"This work was initially inspired by our need to pivot our lab efforts with the onset of the COVID pandemic," Steven A. Sloan, one of the researchers who carried out the study, told Medical Xpress. "We have a longstanding interest in how the fates of neurons and astrocytes are decided during early brain development. It's remarkable, because these cell types share the same progenitors, but those progenitors completely switch their fate from making neurons to astrocytes during a specific developmental time window."
The "gliogenic switch" through which progenitor cells ultimately produce astrocytes has been investigated for decades. Past studies in this area uncovered several molecular changes and secreted molecules that might underpin this switch, ultimately changing the fate of progenitor cells.
"We figured there surely must be more molecular changes that haven't been identified yet," Sloan explained. "Most of studies in this area occurred over a decade ago, and since then there has been an explosion of genomic data and particularly single cell sequencing of the fetal brain. So, when we weren't able to get into the lab to do experiments during COVID, we thought we could mine these datasets and figure out what molecules might be signaling to each other in the developing brain to help switch progenitors fates away from neurogenesis and towards astrogenesis."
The recent work by Sloan and his colleagues was inspired by a previous paper published in Nature Methods, which introduced an algorithm to analyze biological and genomics data. This complex algorithm, called NicheNet, had so far been used to address other research questions that did not relate to the development of astrocytes.
"NicheNet is a complicated algorithm that has a relatively simple explanation," Sloan said. "Computationally, we first had to input RNA-seq (single cell) data of cells that we hypothesized might be sending a signal. In our case, these were the cells in the brain that are present before astrogenesis (mostly, immature neurons). Next, we had to define the cells that might be receiving the signals (progenitors called radial glia)."
After the researchers fed the algorithm relevant RNA-seq data and defined cells that could be receiving signals from them, NicheNet analyzed the data. Essentially, it tried to match molecules with receptors, identifying molecules might have been secreted by sender cells that bind to receptors expressed on receiver cells. This analysis produced a list of possible ligand-receptor pairs involved in the genesis of astrocytes.
"We then took this one step further: instead of just picking some of these candidates, we could filter them by their predicted ability to turn on astrocyte genes," Sloan explained. "This helped a lot, greatly narrowing down our nominated list of candidate molecules. There were some in that list we recognized from the literature, and others that were completely new to us. We thought we could try to find a small cocktail of ligands that might act complementary to each other, with the idea that these molecules probably don't act in isolation."
From the output produced by the NicheNet algorithm, the researchers identified five ligands, each of which would activate a different set of astrocytes genes. Notably, the genes activated by each of these ligands would not overlap with each other.
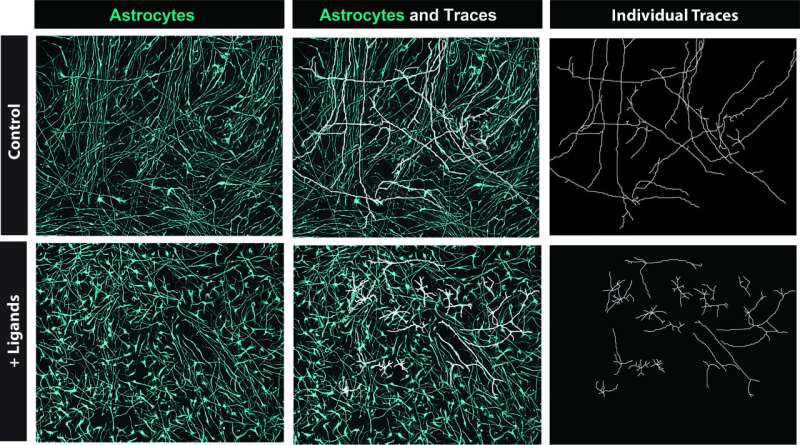
Sloan and his colleagues added these five ligands to organoids (i.e., simplified versions of organs created in laboratory settings) that they were growing in their lab. After 30 days, they applied RNA-sequencing to the organoids to determine if the ligands had influenced the fate of progenitor cells, promoting the genesis of astrocytes.
"We observed a profound upregulation of astrocyte genes and downregulation of neuronal genes," Sloan said. "It really seemed that the ligands were shifting cell fate! We repeated this experiment at multiple timepoints to see when the ligands exert the most effect, and it seems they do this as the receptors increase in expression closer and closer to the gliogenic switch. We also compared adding all five ligands to adding each ligand one-by-one and saw that the cocktail was more potent in inducing this astrocyte commitment."
The researchers carried out some additional experiments to validate the effects they observed in the organoids. Specifically, they introduced the five ligands they identified in human cells and observed what happened.
"Finally, we tried to narrow down which molecular pathways these ligands were activating to push progenitors towards an astrocyte fate," Sloan said. "We queried an assortment of common signaling pathways with a mini-screen and found lots of changes. Yet one that stood out to us was a pathway called mTORC1, which had been previously implicated in astrocyte biology. Therefore, we further validated activation of this pathway upon ligand stimulation and nominate it as a putative regulator of astrocyte development in humans."
By analyzing existing datasets using the NicheNet algorithm, Sloan and his colleagues were able to delineate a hypothesis that they then tested in their lab. Their paper thus reiterates the huge potential of computational methods for conducting research in neuroscience.
"Research groups are continuing to pour out more and more large data with the hopes that they'll lead to new testable hypotheses and experiments," Sloan said. "Here, we're actually following through on that promise. Our study identifies novel gliogenic ligands, but certainly its bigger picture application is the fact that others could use these same datasets to ask hundreds of other questions about how cell-cell communication shapes early brain development."
Future studies could explore the possible role of the ligand-receptor pairs identified by this team of researchers in the further development of astrocytes. In addition, this recent paper could inspire the use of NicheNet or similar algorithms to tackle other research questions related to neuroimmunology, myelination, neuronal specification, the diseased brain and other critical neuroscientific topics.
"Our recent study largely focused on the extrinsic cues that influence astrocyte development," Sloan added. "In the future, we are eager to better understand the intrinsic cellular signals and states that allow a radial glia progenitor to transition from neurogenic to gliogenic fates. This switch seems to be implicated and disrupted in nearly all neurodevelopmental disorders, so a better understanding of the mechanisms that govern this fate decision is quite important."
More information: Anna J. Voss et al, Identification of ligand–receptor pairs that drive human astrocyte development, Nature Neuroscience (2023). DOI: 10.1038/s41593-023-01375-8
Five molecules work together to drive human astrocyte development, Nature Neuroscience (2023). DOI: 10.1038/s41593-023-01391-8
© 2023 Science X Network