This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Cancer-infecting virus 'warms up' cold tumors and improves immunotherapy
Equipping cancer-infecting (oncolytic) viruses with tumor-inhibiting genetic cargo stimulates the immune system and helps immunotherapy to shrink or completely clear aggressive tumors in mice, according to a new study in the Journal of Experimental Medicine led by University of Pittsburgh and UPMC researchers. The results pave the way for clinical trials combining oncolytic viruses with immunotherapy.
Oncolytic viruses are genetically modified viruses that target rapidly dividing tumor cells while avoiding normal cells. Oncolytic viruses were originally designed to directly kill cancer cells, but researchers later noticed that they also stimulated the immune system, suggesting that they could be coupled with other cancer therapies such as immune checkpoint inhibitors, which remove the brakes on the immune system so that T cells can recognize and attack tumors.
"Immune checkpoint inhibitors work only in 'hot' tumors, which have already been infiltrated by T cells," said senior author Greg Delgoffe, Ph.D., associate professor of immunology at Pitt's School of Medicine and director of the Tumor Microenvironment Center at UPMC Hillman Cancer Center. "Oncolytic viruses can help 'warm up' cold tumors, so they have amazing potential to work hand-in-hand with immunotherapy, but they haven't yet lived up to that promise."
According to lead author Kristin DePeaux, a graduate student in Delgoffe's lab, the problem is that many patients' tumors do not respond to oncolytic viruses.
"There's been a lot of interesting lab-based research on oncolytic viruses, but it hasn't translated to the clinic," she said. "We wanted to understand the mechanisms behind tumor resistance to these viruses to see what we can do to help patients."
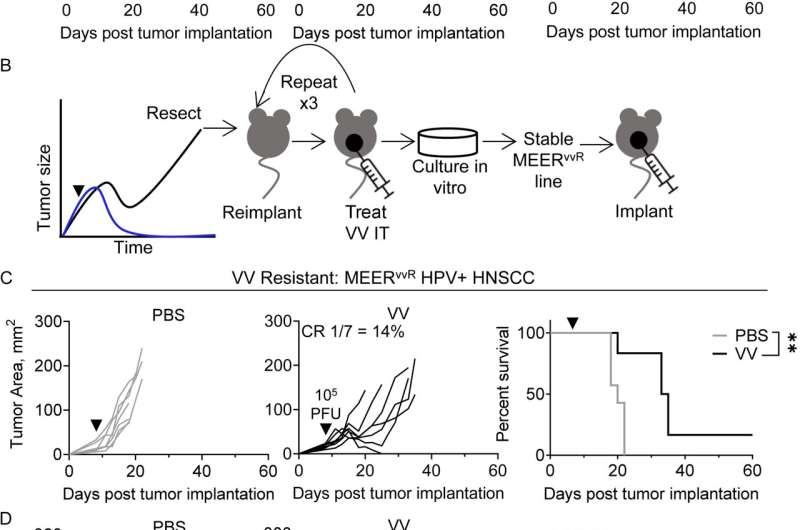
The researchers first developed a head-and-neck squamous cell carcinoma (HNSCC) cell line that is very sensitive to an oncolytic virus called vaccinia. Tumors injected with the virus regress after a single dose. They also developed a second cancer cell line that was otherwise identical but resistant to vaccinia.
After injecting both types of cells into mice and comparing immunological differences in the tumors that grew, they found that resistance to vaccinia was driven by high levels of a signaling protein called TGF-β, which is known to promote cancer growth by suppressing the immune environment.
Partnering with Andrew Hinck, Ph.D., professor of structural biology at Pitt, the team next engineered vaccinia to carry a gene encoding a TGF-β inhibitor.
"TGF-β inhibitors are very potent. They've been tried in the clinic, but they're usually toxic because they're delivered systemically," said Delgoffe. "What's really cool about using oncolytic viruses is that they deliver this cargo directly to the tumor microenvironment, so it's very targeted and a much safer way to treat."
When they injected the modified vaccinia into mice with vaccinia-resistant HNSCC, the tumors shrank or, in about 50% of mice, completely cleared, greatly increasing survival compared to animals that received the control virus, which didn't carry the TGF-β inhibitor. Importantly, the treatment didn't cause any autoimmune or toxicity-related side effects.
Next, the researchers tested whether the modified viruses could work similarly in a highly aggressive form of melanoma that is resistant to anti-PD1 immune checkpoint inhibitors. Animals that received no treatment, anti-PD1 or control vaccinia all died within about 24 days, while about 20% of those that received the modified virus had complete clearance of the tumor.
The most dramatic results occurred when modified vaccinia was combined with anti-PD1. In 67% of mice, tumors completely cleared, and survival was greatly extended.
Delgoffe and his team hope that a version of their modified vaccinia virus, which they've licensed to Kalivir Immunotherapeutics, could soon be ready to test in human clinical trials as an adjuvant for immune checkpoint inhibitors in patients who haven't responded to these immunotherapies.
Additional authors of the study were Dayana Rivadeneira, Ph.D., Victoria Dean, William Gunn, Tianhong Yao, Drew Wilfahrt, Ph.D., Cynthia Hinck, Ph.D., and Lukasz Wieteska, Ph.D., all of Pitt; Konstantinos Lontos, M.D., Ph.D., of the University of Texas; McLane Watson, Ph.D., of the Van Andel Institute; and Stephen Thorne, Ph.D., of Kalivir Immunotherapeutics.
More information: Kristin DePeaux et al, An oncolytic virus–delivered TGFβ inhibitor overcomes the immunosuppressive tumor microenvironment, Journal of Experimental Medicine (2023). DOI: 10.1084/jem.20230053