This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
'Anti-tangle' molecule could aid search for new dementia treatments, say scientists
Scientists have identified a molecule that can prevent tangling of a brain protein that is linked to diseases such as Parkinson's. The findings may provide insights into new ways of treating or diagnosing the early stages of dementia.
Alpha-synuclein, a protein found in brain cells, is commonly associated with neurodegenerative diseases such as Parkinson's, a debilitating neurological disorder affecting millions worldwide.
Like all proteins, it is made up of a long strand of molecules called amino acids. When it's made, this strand folds in on itself to form a complex but precise 3D shape, made up of sub-structures and loops.
In healthy individuals, alpha-synuclein interacts with cell membranes where it plays a role in how brain cells (neurones) communicate with each other, but as a person ages, the 3D shape of the protein can malform, or "misfold," causing it to start sticking together to form toxic clumps in the brain.
Over time these clumps continue to stack, forming fibers that can interfere with the protein's normal role, eventually killing brain cells, contributing to the development of Parkinson's and related dementia diseases.
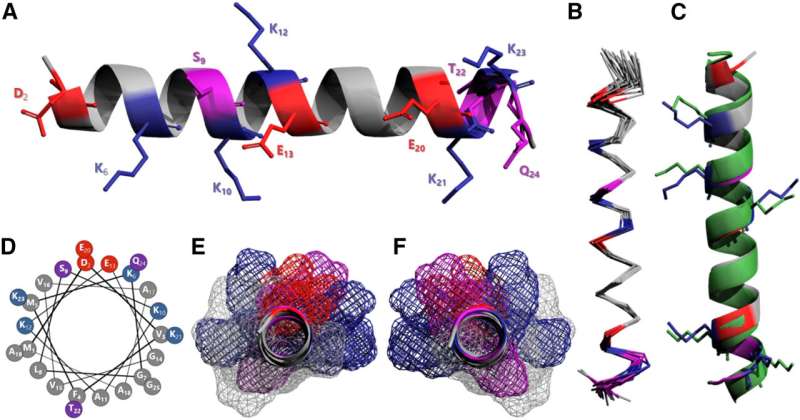
A team of scientists at the Universities of Bath and Bristol took a protein fragment, or peptide, from one end of the alpha-synuclein protein strand and mixed it with samples of the full-length alpha-synuclein protein.
They found that the fragment prevented misfolding in vitro, by stabilizing its normal structure to prevent it from tangling, forming clumps and disrupting the cell membrane.
Published in the journal Cell Reports Physical Science, this research opens up new avenues for therapeutic development, potentially in the future leading to drugs that can target and disrupt alpha-synuclein misfolding, ultimately preventing or slowing down the progression of diseases like Parkinson's.
Professor Jody Mason, from the Department of Life Sciences at the University of Bath, led the research. He said, "Currently drug options for Parkinson's only treat the symptoms of the disease, often by restoring the lost communication between neurones."
"Unfortunately, these treatments have side effects, are less effective over time, and do not slow the underlying pathology."
"If we could diagnose before symptoms present, and block alpha-synuclein misfolding at the very earliest stages that are prior to clumping, we could slow disease progression instead of just managing the symptoms after the damage has already been done."
"This in vitro study has demonstrated the potential of this peptide, which we can use as a template to design new drugs that treat the earliest stages of these terrible diseases."
The researchers are now looking for funding to continue their research and test out different variations of the peptide on brain cells grown in the lab, before identifying suitable candidate molecules for further drug development.
Professor Mason said, "Our research is still at the early stages, but we hope that it's a stepping stone towards new treatments for neurodegenerative diseases."
More information: Richard M. Meade et al, An N-terminal alpha-synuclein fragment binds lipid vesicles to modulate lipid-induced aggregation, Cell Reports Physical Science (2023). DOI: 10.1016/j.xcrp.2023.101563