This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists reveal cellular changes unique to early Alzheimer's disease
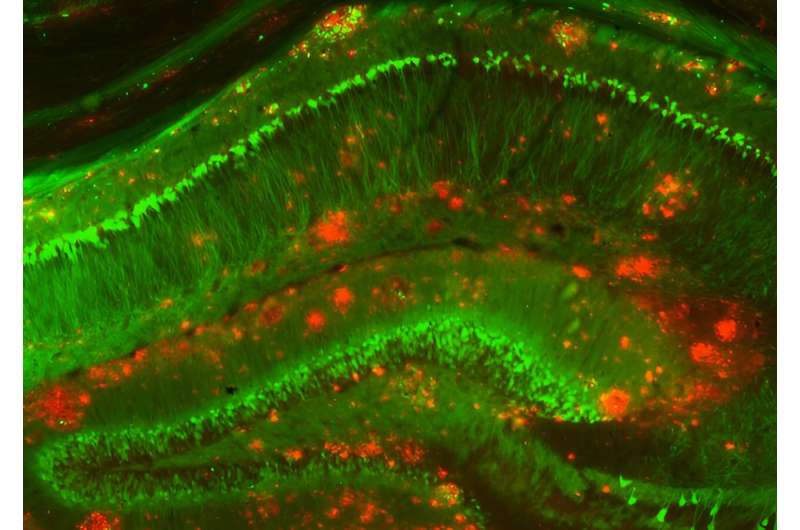
Most Alzheimer's disease research on human brain tissue has studied postmortem samples, making it difficult for scientists to discern the earliest events in the brain that might have triggered the buildup of plaques and the death of neurons. Knowing the molecular changes in neurons, glia, and other brain cells around plaques during the early phases of the disease could help scientists design treatments that work best when given early.
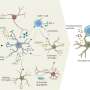
Now, in a study appearing in Cell, a team led by researchers at the Broad Institute of MIT and Harvard has analyzed an assembly of rare brain tissue samples from 52 living patients with varying degrees of other Alzheimer's-related changes in the brain—including 17 individuals who were later clinically diagnosed with the disease. The scientists identified a suite of changes in cells unique to the early stages of Alzheimer's, including some not seen before in animal studies.
The team discovered a brief hyperactive state in a specific group of neurons that was associated with their death in later stages of the disease, and also increased inflammatory processes in immune cells called microglia as the disease progressed.
Neurons are thought to produce the plaque-forming protein called amyloid beta, and the researchers found evidence for this in their data. They also found for the first time that another brain cell type, oligodendrocytes, which produce insulating sheaths around nerve fibers in the brain, may also contribute to plaque formation. A better understanding of how these cells spur the growth of plaques could one day help researchers identify new targets for Alzheimer's drugs.
The study is a result of close collaboration with Ville Leinonen, a neurosurgeon and professor from the University of Eastern Finland who has spent more than a decade collecting and studying brain tissue samples from patients who underwent routine surgeries for other neurological conditions and agreed to provide a small amount of brain tissue and other samples for research.
"This was just a really rich opportunity to peer into the actual workings of cells with minimal artifacts and see what they're doing in the context of amyloid," said Evan Macosko, senior author on the study, an institute member at the Broad, and associate professor and attending psychiatrist at Massachusetts General Hospital.
Beth Stevens, an institute member at the Broad, an associate professor at Harvard Medical School, and a research associate in neurobiology at Boston Children's Hospital, was a co-author on the study.
"This was a really great synergy of computational, wet lab, and clinical work," said Tushar Kamath, co-first author on the study and an MD/Ph.D. student in Macosko's lab when the study began. "It took a decade of neurosurgeries, patient involvement, thoughtful analyses, and really useful experiments. We couldn't have done this study if any one of those things hadn't happened."
Vahid Gazestani, a research scientist in Macosko's lab when the study began and now a senior computational scientist at Johnson & Johnson, was the other co-first author on the study.
An early look
Cells—and in particular, neurons—change rapidly after losing their supply of oxygen postmortem, potentially making it difficult for scientists to accurately study how they work when just looking at postmortem samples.
Macosko was discussing these limitations with other researchers at a neuroscience meeting five years ago when a colleague suggested he speak with Leinonen.
Leinonen was studying early Alzheimer's disease and normal pressure hydrocephalus (NPH), a neurological disorder characterized by excess fluid around the brain. He had a collection of brain tissue samples obtained from NPH patients during routine surgeries to reduce excess brain fluid. He'd collected other samples such as cerebrospinal fluid from the same patients, and followed the cohort over time, recording clinical data such as whether or not patients developed Alzheimer's.
Macosko knew samples from these living patients presented a rare chance to observe cells exposed to the initial stages of Alzheimer's pathology.
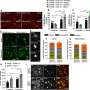
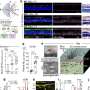
Over the next few years, his team used single-nucleus RNA sequencing, which maps gene expression in individual cell nuclei, to analyze the brain tissue. By cross-checking this data with Leinonen's clinical notes and integrating with previous single-cell postmortem and mouse studies, the researchers identified key changes in various cell types at the early stages of the disease.
"These samples gave us a high-quality anchor to reliably identify cell types in all the other datasets," said Gazestani. "This whole integrative analysis was feasible because of the quality and the depth of the dataset that we had."
Capturing cell changes
Researchers have long assumed that neurons produce the amyloid protein, but this has been difficult to prove in human tissue. In the new study, Macosko's team found that neurons showed gene expression signatures associated with amyloid production. For the first time, they also observed the same signature in oligodendrocytes.
"This is exciting because there are lots of ways you can make amyloid accumulate in the brain in mouse studies, but now we've seen what's actually happening in a human," Macosko said.
The researchers also observed a hyperactive population of neurons in the upper layer of the brain. This group of neurons die early in the disease, and Macosko thinks that future studies could show that this hyperactivity precipitates more widespread loss of neurons in patients.
Finally, the researchers identified microglia—cells that help clear the amyloid-beta peptide from the brain—that were functioning in two different kinds of activated states. Some of these states have not been detected previously in microglia in animal models, though the researchers recently confirmed several of these diverse states in induced pluripotent stem cells.
In the present study, the team found cells in one of these states in tissue from both patients with Alzheimer's and those with Parkinson's—a finding that could provide clues about similarities between the conditions.
In the future, Macosko and Stevens plan to identify proteins that are correlated with these cell states in pairs of blood and cerebrospinal fluid samples, which could serve as markers of disease progression. They hope, too, that other researchers will use their approach to analyze datasets from different kinds of samples and sequencing methods together.
"One of the biggest challenges in this field is the fact that every data set is analyzed separately," Macosko said. "We're hopeful that this cohort can be useful as a reference for others to align and integrate their own data so that it becomes easier to compare and contrast different diseases or cohorts."
Already, the findings have provided a launching point for a new research effort coordinated by Stevens, Macosko, and members of the International Neuroimmune Consortium, which brings together researchers from the Broad, the University of Eastern Finland, Stanford University, the UK Dementia Research Institute, and others. The collaboration aims to determine how neuro-immune interactions contribute to cell vulnerability and neurodegeneration in Alzheimer's and other brain diseases.
"This cohort provides us with ground truth on changes in neurons and glia in the brains of living patients at early stages of disease progression," said Stevens. "That could lead to new neuroimmune biomarkers and improvements in biomarkers discovery in the blood and CSF."
More information: Vahid Gazestani et al, Early Alzheimer's disease pathology in human cortex involves transient cell states, Cell (2023). DOI: 10.1016/j.cell.2023.08.005