This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers identify peptides produced by the proteasome that degrade damaged proteins

The waste system of living cells, the proteasome, not only shreds disused or damaged proteins, it also supports the immune system by recognizing virally infected or cancerous cells by producing protein fragments, so-called immunopeptides.
In an international collaboration, researchers led by Juliane Liepe at the Max Planck Institute (MPI) for Multidisciplinary Sciences have now simulated protein degradation by the proteasome in the laboratory and identified and quantified the peptides produced. In the future, the resulting data set could help predict immunopeptides and develop new vaccines against infectious diseases or cancer.
The research is published in the journal Nature Communications.
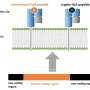
The circular economy and recycling are hot topics in today's world. Living cells have already perfected what often does not run smoothly in our everyday lives. The proteasome—the cellular recycling plant—breaks down proteins that are no longer needed or defective. The resulting peptides can then be used as components for new proteins or brought to the cell's surface, where they serve as "signal flags" for our immune system.
Certain immune cells check whether these peptide "flags" are endogenous or foreign. "They recognize the unfamiliar peptides and know: Something is wrong here! Cells of our immune system can thus distinguish between healthy and infected or cancerous cells," explains Juliane Liepe, research group leader at the MPI for Multidisciplinary Sciences. The immune system can then destroy the infected or cancer cells.
Recycling in focus
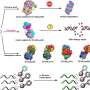
However, the proteasome not only breaks down proteins into smaller peptides. It can also reassemble several of them. This process is called peptide splicing. A considerable number of the peptides presented to the immune system are such spliced variants.
Liepe's Quantitative and Systems Biology research group is particularly interested in which spliced peptides contribute to immune defense and according to which rules the proteasome generates them. "For example, if we know the spliced tumor peptides that cancer cells present to the immune system, it may be possible to use these for immunotherapies in future. To do this, we need to know which specific peptides the proteasome produces and which meaning they bear," explains the bioinformatician.
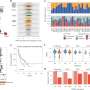
To better understand the process of peptide splicing, the researchers used lab experiments to demonstrate how the proteasome degrades entire proteins and determined the produced peptides qualitatively and quantitatively.
For this extensive project, the research team collaborated closely with scientists from the Francis Crick Institute and King's College London (U.K.), the National University of Singapore, and teams led by Stefan Becker, Ashwin Chari, and Henning Urlaub at the MPI. Together, they were able to generate the largest known data set of peptides produced from proteins by the proteasome under laboratory conditions.
In addition to biochemical experiments and mass spectrometry methods, the researchers used computational methods, partly based on machine learning. "Among other things, we developed new computer programs to identify and quantify non-spliced and spliced peptides," Liepe describes.
Not random at all
With help of their data, the scientists have uncovered some of the proteasome's secrets. Neither the cutting nor the splicing of peptides happens by chance. "We found that the proteasome prefers to process certain protein areas," explains Michele Mishto, head of the London research group.
"This is partly due to the proteasome's preferences for specific sequences of protein components," Wai Tuck Soh reports.
"We also discovered clear features that distinguish spliced from non-spliced peptides," adds Hanna Rötschke, who—alongside Soh and John Cormican—is a first author of the work published in Nature Communications.
"If we know how the proteasome generates immunopeptides, we can predict peptide splicing. In future, these predictions could, in turn, help develop novel vaccines against cancer or infectious diseases," states Liepe.
More information: Wai Tuck Soh et al, Protein degradation by human 20S proteasomes elucidates the interplay between peptide hydrolysis and splicing, Nature Communications (2024). DOI: 10.1038/s41467-024-45339-3