This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
How cancer cells harness energy to drive disease progression
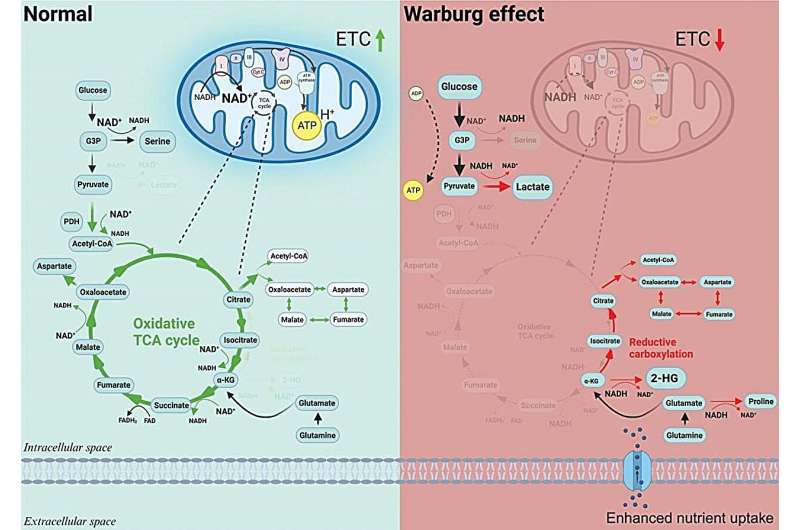
Researchers have revealed crucial insights into how the Warburg effect causes the dedifferentiation of cancer cells through epigenetic reprogramming. This discovery potentially opens up new avenues for cancer treatments that target cellular metabolism.
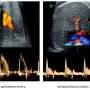
A century after Otto Warburg first described the phenomenon where cancer cells undergo glycolysis instead of oxygen respiration—even in oxygen-rich environments—the so-called Warburg effect continues to be a fundamental element in understanding cancer metabolism and a key factor in the detection of tumors through PET scans.
Researchers have now unveiled new details on how this effect significantly influences cancer cell behavior and treatment outcomes.
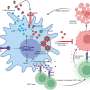
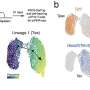
An editorial published in Cancer Biology & Medicine, December 2023, has demonstrated how the Warburg effect, a phenomenon where cancer cells prefer glycolysis over oxygen respiration even when ample oxygen is available, is crucially linked to cancer progression. This study illustrates that the Warburg effect represents mitochondrial dysfunction, which in turn triggers metabolic reprogramming within the cancer cells.
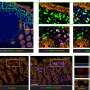
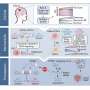
Recent research advancements have established that when mitochondria are impaired, they fail to function as normal, which triggers a chain of metabolic reprogramming within the cells. This reprogramming is not just a shift in energy production but significantly impacts the epigenetic landscape of the cells.
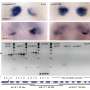
These epigenetic changes lead to dedifferentiation, where specialized, mature cells revert to a less specialized, stem-like state. This process is critical as it allows cancer cells to multiply rapidly and resist conventional treatments, leading to aggressive tumor growth and increased malignancy.
Furthermore, recent studies have highlighted how mutations accumulate in mitochondrial DNA as cells age, exacerbating mitochondrial dysfunction. This accumulation is linked directly to the metabolic shifts that promote the cancerous phenotype.
By connecting these dots, the review provides a clear pathway showing how mitochondrial dysfunction through the Warburg effect leads to changes in gene expression and cell behavior via epigenetic mechanisms.
Dr. Jiangbin Ye, lead researcher and an Assistant Professor at Stanford University, stated, "Our study marks a significant breakthrough in understanding the Warburg effect's role in cancer progression. By linking metabolic changes to epigenetic modifications, we can now target these pathways to potentially reverse the dedifferentiation of cancer cells."
The research not only revisits and substantiates Otto Warburg's theories but also opens exciting new avenues in the fight against cancer, blending metabolic manipulation with traditional treatments to potentially enhance patient outcomes significantly.
More information: Haowen Jiang et al, The Warburg effect drives dedifferentiation through epigenetic reprogramming, Cancer Biology & Medicine (2024). DOI: 10.20892/j.issn.2095-3941.2023.0467