This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
PKMYT1, a potential 'Achilles heel' of treatment resistant ER+ breast cancers with the poorest prognosis
Up to 80% of breast cancer deaths occur in patients with tumors that express estrogen receptor-alpha. Although these estrogen receptor-positive (ER+) breast cancers often initially respond to standard treatment that combines endocrine therapies with CDK4/6 inhibitors, drug resistance often develops leading to lethal metastatic disease that spreads from the breast and does not respond to available treatments.
Looking to identify new vulnerabilities in this type of cancer that could lead to improved therapies, researchers at Baylor College of Medicine and collaborating institutions focused on studying proteins produced by ER+ breast cancers that are resistant to the combination therapy. Specifically, they searched for enzymes called kinases, whose expression is typically altered in cancer. Their promising results are now published in Molecular Cancer Therapeutics.
"Kinases have proven to be effective therapeutic targets for cancer and there are many inhibitors of these enzymes that are already approved by the Food and Drug Administration for human use that could be tested for their potential therapeutic value in breast cancers," said corresponding author Dr. Charles Foulds. "The challenge was to identify the kinase among hundreds of kinases in these treatment-resistant tumors that would help us turn the tide in the fight against this cancer."
KIPA helps find the needle (select kinase) in a haystack (of hundreds of kinases)
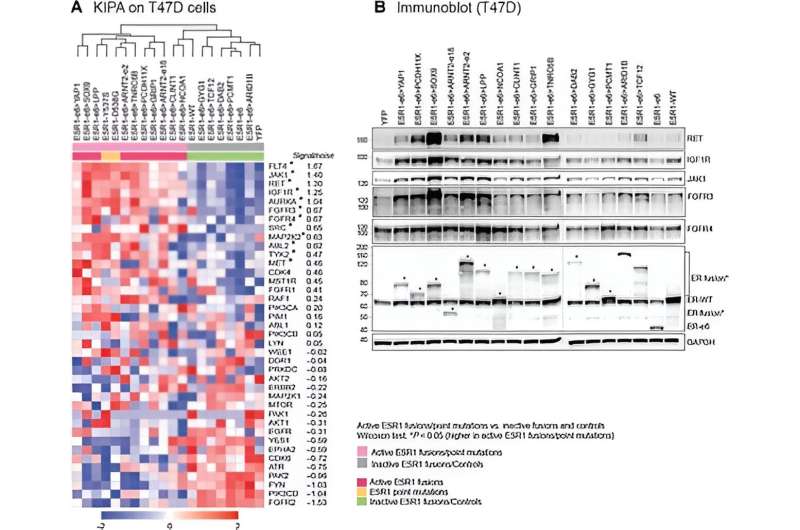
The researchers had previously developed a laboratory method they called kinase inhibitor pull-down assay (KIPA) that significantly sped up the process of kinase identification among hundreds of potential candidates.
Working with 22 patient-derived xenografts (PDXs), human breast cancer tumors grown in immune-compromised mouse models, the team used KIPA to identify and then compare the kinases produced by tumors whose growth depended on the hormone estradiol, with the kinases produced by tumors whose growth was independent of that estrogen.
"The goal was to identify the difference between the estrogen-dependent group, which clinically represents cancers that respond to endocrine therapy (this therapy makes the estrogen the tumor needs to grow unavailable, therefore reducing cancer growth), versus tumors whose growth is independent of estrogen, which clinically, typically correlates with the group that does not respond to endocrine therapy," said first author Dr. Anran Chen. "Our comprehensive analyses showed that the protein kinase, membrane-associated tyrosine/threonine one or PKMYT1, was our best candidate."
Furthermore, patient clinical samples and breast cancer cell lines with high PKMYT1 mRNA levels, an indicator of PKMYT1 gene expression, were associated with resistance to both endocrine therapy and CDK4/6 inhibition. "These findings suggested that a high PKMYT1 level could be an indicator for treatment response in ER+ breast cancer tumors," Chen said. "Because this kinase is involved in the regulation of cell division, we decided to investigate the effect a PKMYT1 inhibitor in clinical development would have on cancer growth."
Therapeutic potential
Working with PDXs, organoids (mini tumors grown in the lab), cell lines and clinical samples, the researchers discovered that combining the PKMYT1 inhibitor lunresertib (also called RP-6306) with the chemotherapy drug gemcitabine selectively and synergistically reduced the viability of ER+ breast cancer cells that were resistant to endocrine therapy and the CDK4/6 inhibitor palbociclib and also lacked a functional tumor suppressor protein called p53. The absence of functional p53 disturbs cell cycle regulation in response to DNA damage, leading to poor clinical outcomes in ER+ breast cancer.
"We were excited to find that combining a PKMYT1 inhibitor with gemcitabine led to DNA damage and cell death in p53 dysfunctional cancer cells grown in the lab, and markedly reduced the size of PDX organoids grown in the lab and of tumors grown in immune-compromised mice, compared to treatment with either drug alone," Foulds said.
"Our study shows that PKMYT1 is not only a potential marker for these tumors' response to endocrine therapy and CDK4/6 inhibitor treatment, but also has clinical potential as a therapeutic target for treating drug resistant, mutant p53 ER+ breast cancer," Chen said. "Our pre-clinical findings support further investigations to determine the value of this novel potential therapy to treat one of the most challenging human cancers."
More information: Anran Chen et al, PKMYT1 is a Marker of Treatment Response and a Therapeutic Target for CDK4/6 Inhibitor-Resistance in ER+ Breast Cancer, Molecular Cancer Therapeutics (2024). DOI: 10.1158/1535-7163.MCT-23-0564