This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists find 'Goldilocks' binding strength determines anti-cancer T-cell efficacy and fate
Immunotherapy, treatments that reinvigorate immune cells' anti-cancer activity or reprogram T cells to target cancer, has shown promise in treating leukemias but has not yet been realized in solid tumors. One reason for the stymied success is the conversion of potential cancer-killing T cells into an inactive "exhausted" state near the tumor.
St. Jude Children's Research Hospital scientists found that how tight a parental T cell grabs a cancer protein determines if its daughter cells will be anti-cancer effectors or exhausted. The findings, which have broad implications for improving immunotherapy, were published today in Nature Immunology.
T cells are the primary anti-cancer immune cells that detect and destroy cancer. Each T cell has a special detection protein on its surface, the T-cell receptor, which binds to a single cancer-related protein, a process that stimulates the immune cell to destroy the cancer.
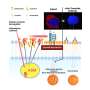
The St. Jude team showed that a "medium" binding strength between the T-cell receptor and the cancer protein results in the best anti-cancer activity in mouse models.
"We found that T-cell receptor binding and signal strength sets up a Goldilocks scenario," said corresponding author Benjamin Youngblood, Ph.D., St. Jude Department of Immunology. "Too much stimulation will drive the T cells to a terminal state, limiting their ability to fight cancer. But too little stimulation may also cause them to become dysfunctional. You want to hit that 'just right' state."
Early T-cell activation determines anti-cancer potential
Anti-cancer potential is decided early in a T cell's life when it engages with other immune cells that alert the T cell of a tumor's presence. It is now well-appreciated that uncontrolled tumors can indeed be recognized by patients' T cells but have lost their ability to kill the tumor and subsequently become exhausted from their fight.
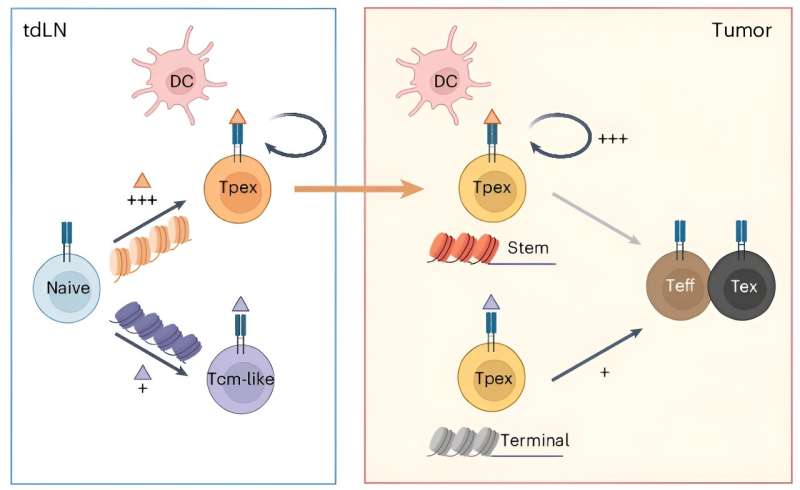
Efforts to understand this exhausted state have revealed that the T cell was, in fact, capable of killing the tumor during the initial immune response but had subsequently lost its killing potential. Because these "progenitor" T cells retain tremendous anti-tumor potential, there is great interest in determining how to maintain them and the mechanisms that promote their transition into the non-functional exhausted T cell.
The St. Jude investigators discovered the tightness of the bond between a progenitor T cell and a tumor-surveying immune cell determines the functionality of the progenitor's offspring. If binding is too tight or loose, the progenitor T cells develop into exhausted cells. Only when the parental cell's T-cell receptor managed a Goldilocks middle-ground binding strength were cancer-killing effector cells created.
"It really is a beautiful and simple mechanism," Youngblood said. "Optimal stimulation enables sustained contact with a cell that can provide healthy or good signals, whereas, with low stimulation, the T cell can't stay in contact with the cancer-protein presenting cells, so it physically moves then enters that more suppressive toxic state."
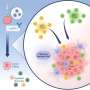
The authors specifically found that optimal engagement needed to be between T cells and dendritic cells, a different type of white blood cell. Dendritic cells serve a critical role in cancer surveillance. After encountering the tumor, dendritic cells presented pieces of cancer-related proteins to the parental T cell.
When T-cell receptor binding to the presented cancer protein fell within the Goldilocks strength range, the researchers saw increased T-cell proliferation and activation in vitro and in vivo. If the binding was too weak, the parental cell moved away from the dendritic cell and experienced its own dysfunction.
The scientists identified the genes and epigenetic modifications associated with each outcome, providing a resource to improve future T-cell–based therapies.
Improving anti-cancer T-cell therapies
Current immunotherapies rely heavily on T cells. One such group of therapies, immune checkpoint inhibitors or blockade, prevent the tumor from turning T cells off. The approach has shown limited success when the pool of progenitor T cells has been depleted and only fully exhausted T cells remain. Incorporating the findings from Youngblood's lab into the development of novel therapies, as well as the timing of their administration, could improve efficacy.
"The progenitor T-cell population is the one that responds to checkpoint blockade, not exhausted T cells," Youngblood said. "If you don't have that population, you never get a therapeutic response to checkpoint blockade. Understanding what controls that transition between progenitor to the dysfunctional exhausted state is what's going to allow us to move forward with T-cell based-immunotherapies in the solid tumor setting."
In addition to checkpoint inhibitors, the results could help improve immunotherapy that reprograms a patient's immune cells with an artificial T-cell receptor. This artificial chimeric antigen receptor (CAR) targets a chosen cancer-related protein (antigen), leading to T-cell killing of the tumor. The findings provide evidence that choosing CAR targets could be improved.
"We need to pay closer attention to T-cell stimulation as they enter the tumor microenvironment," Youngblood said. "It's not good enough just to pick any tumor antigen. We must pick a tumor antigen that gives an optimal signal when picking antigens for approaches such as therapeutic vaccination or CAR T cells. We now know to ask: Are they too strong? Are they too weak? Or are they just right?"
More information: Xin Lan et al, Antitumor progenitor exhausted CD8+ T cells are sustained by TCR engagement, Nature Immunology (2024). DOI: 10.1038/s41590-024-01843-8