Inhibiting formation of a branched sugar molecule could lead to new treatments for multiple sclerosis
Multiple sclerosis is a debilitating condition that involves the degeneration of myelin—the fatty tissue that insulates nerve fibers and helps them to conduct impulses. This process, called demyelination, can lead to deficits in sensation, movement and thought processes, depending on exactly which nerve fibers are affected. Replacing lost myelin is a promising approach for treating multiple sclerosis and related diseases, but the mechanisms underlying demyelination and remyelination remain poorly understood.
In research that opens up an encouraging avenue for the development of new treatments, Naoyuki Taniguchi and colleagues from the RIKEN–Max Planck Joint Research Center for Systems Chemical Biology have shown that remyelination is inhibited by sugar molecules called branched O-mannosyl glycans.
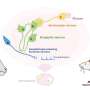
Taniguchi and his colleagues genetically engineered a strain of mice carrying mutations in the gene encoding an enzyme called N-acetylglucosaminyltransferase-IX (GnT-IX), which catalyzes the branching of O-mannosyl glycan sugars on proteins in the brain. Using these mice, the researchers found that GnT-IX acts on a specific brain protein called receptor protein tyrosine phosphatase ? (RPTP?), which has previously been shown to play a critical role in demyelination.
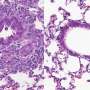
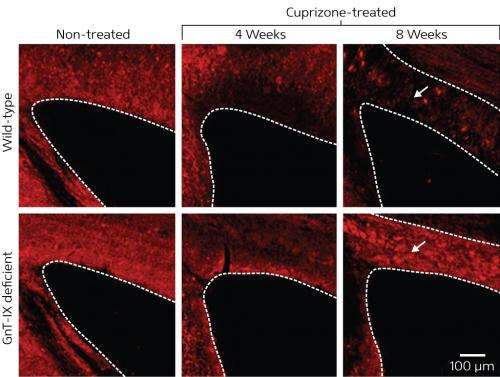
Next, the research team fed normal and mutant mice a diet containing the neurotoxin cuprizone, which normally induces demyelination. Over the course of eight weeks, the normal mice were found to have experienced gradual demyelination of the corpus callosum—a major tract of white matter connecting the two hemispheres of the brain (Fig. 1). By contrast, although myelin in the corpus callosum had degraded by four weeks in the mutants, myelination had markedly increased by the eight-week mark, suggesting that the defect in the GnT-IX gene enhanced remyelination.
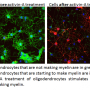
Further experiments revealed that cuprizone treatment can activate non-neuronal cells called astrocytes into a disease state, which leads them to express RPTP? containing branched O-mannosyl glycans. In wild-type mice, activated astrocytes express these branched O-mannosyl glycan molecules, which inhibit remyelination. In mice with a defective GnT-IX gene, however, astrocytes are rarely activated, and the absence of the branched O-mannosyl glycans allows the differentiation of myelin cells and the remyelination of nerve fibers in the corpus callosum.
"We would like to unveil the molecular mechanism by which branched O-mannosyl glycan activates astrocytes," says Taniguchi. "Understanding the underlying mechanism is important for developing a drug to treat multiple sclerosis."
The team next plans to screen for a GnT-IX inhibitor that attenuates astrocyte activation. "The difficult thing is that the drug has to pass through the blood–brain barrier, so collaboration with clinicians will be important," Taniguchi notes.
More information: Kanekiyo, K., et al. Loss of branched O-mannosyl glycans in astrocytes accelerates remyelination, The Journal of Neuroscience 33, 10037–10047 (2013). dx.doi.org/10.1523/JNEUROSCI.3137-12.2013