Protein controlling glucose metabolism also a tumor suppressor
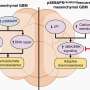
A protein known to regulate how cells process glucose also appears to be a tumor suppressor, adding to the potential that therapies directed at cellular metabolism may help suppress tumor growth. In their report in the Dec. 7 issue of Cell, a multi-institutional research team describes finding that cells lacking the enzyme SIRT6, which controls how cells process glucose, quickly become cancerous. They also found evidence that uncontrolled glycolysis, a stage in normal glucose metabolism, may drive tumor formation in the absence of SIRT6 and that suppressing glycolysis can halt tumor formation.
"Our study provides solid evidence that SIRT6 may function as a tumor suppressor by regulating glycolytic metabolism in cancer cells," says Raul Mostoslavsky, MD, PhD, of the Massachusetts General Hospital (MGH) Cancer Center, senior author of the report. "Critically, our findings indicate that, in tumors driven by low SIRT6 levels, drugs that may inhibit glycolysis – currently a hot research topic among biotechnology companies – could have therapeutic benefits."
The hypothesis that a switch in the way cells process glucose could set off tumor formation was first proposed in the 1920s by German researcher Otto Warburg, who later received the Nobel Prize for discoveries in cellular respiration. He observed that, while glucose metabolism is normally a two-step process involving glycolysis in the cellular cytoplasm followed by cellular respiration in the mitochondria, in cancer cells rates of glycolysis are up to 200 times higher. Warburg's proposition that this switch in glucose processing was a primary cause of cancer did not hold up, as subsequent research supported the role of mutations in oncogenes, which can spur tumor growth if overexpressed, and tumor suppressors, which keep cell proliferation under control. But recent studies have suggested that alterations in cellular metabolism may be part of the process through which activated oncogenes or inactivated tumor suppressors stimulate cancer formation.
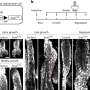
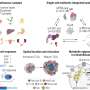
A 2010 study led by Mostoslavsky found that the absence of SIRT6 – one of a family of proteins called sirtuins that regulate many important biological pathways – appears to "flip the switch" from normal glucose processing to the excess rates of glycolysis seen in cancer cells. The current study was specifically designed to investigate whether SIRT6's control of glucose metabolism also suppresses tumor formation. The research team first showed that cultured skin cells from embryonic mice lacking SIRT6 proliferated rapidly and quickly formed tumors when injected into adult mice. They also confirmed elevated glycolysis levels in both cells lacking SIRT6 and tumor cells and found that formation of tumors through SIRT6 deficiency did not appear to involve oncogene activation.
Analysis of tumor samples from patients found reduced SIRT6 expression in many – particularly in colorectal and pancreatic tumors. Even among patients whose tumors appeared to be more aggressive, higher levels of SIRT6 expression may have delayed or, for some, prevented relapse. In a mouse model programmed to develop numerous colon polyps, the researchers showed that lack of intestinal SIRT6 expression tripled the formation of polyps, many of which became invasive tumors. Treating the animals with a glycolytic inhibitor significantly reduced tumor formation, even in the absence of SIRT6.
"Our results indicate that, at least in certain cancers, inhibiting glycolytic metabolism could provide a strong alternative way to halt cancer growth, possibly acting synergistically with current anti-tumor therapies," says Mostoslavsky, an assistant professor of Medicine at Harvard Medical School. "Cancer metabolism has only recently emerged as a hallmark of tumorigenesis, and the field is rapidly expanding. With the current pace of research and the speed at which some basic discoveries are moving into translational studies, it is likely that drugs targeting cancer metabolism may be available to patients in the near future."
More information: Sebastián et al., Cell, "The Histone Deacetylase SIRT6 Is a Tumor Suppressor that Controls Cancer Metabolism," Dec.7, 2012, dx.doi.org/10.1016/j.cell.2012.10.047