July 19, 2013 report
Myelin exploits phase transitions to drive it's assembly
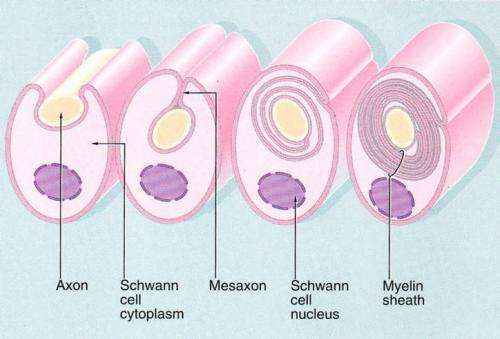
The ability to construct complex myelin sheaths around axons is one of the greatest vertebrate inventions since the hinged jaw.
According to one theory, as placoderms (the first hinge-jawed fish) started evolving into creatures of formidable size, the usual strategy for increasing nerve impulse speed, namely using fatter axons, was not called upon. Instead, the demands for speed in critical pathways, like their now 10-fold longer oculomotor system, were met by developing elaborate myelin structures which wrap around the axons to insulate them. But how do these compact, crystalline structures form, and from where does the energy to do so derive? Some of the first clues towards answering these questions have come from researchers in Göttingen, Germany, who have proposed that myelin membrane assembly is driven by a phase transition of Myelin Basic Protein (MBP) into a cohesive protein network. Their latest work, just published in PLoS Biology, suggests that as myelin membranes zipper together, proteins other than MBP are extruded by specific hydrophobic interactions similar to the beta sheet structures observed in amyloid assembly.
Myelin is around 80% lipid by dry weight, and MBP makes up much of the remaining mass. The researchers tracked the diffusion of optically-labelled MBP in cultured myelinating cells, to investigate its assembly. Beyond a critical concentration, MBP was found to rapidly polymerize into a meshwork which acts to squeeze out most other proteins. The formation of these large oligomeric complexes then acts to draw opposing cytoplasmic membrane surfaces together. The researchers found that this transition was reversible upon pH manipulations.
The researchers were also able to show that the inherent capacity to induce phase transitions was dependent on hydrophobic interactions mediated through phenylalanine residues at specific amino acid positions in MBP. Converting these residues to serines through genetic manipulations abolished the transitions in a fashion similar to that seen in amyloid beta sheet assembly.
These kinds of studies are a refreshing diversion from the more typical "who-binds-who" studies which continue to dominate much of cell biology. The authors note that while there is now a wealth of knowledge about specific molecular interaction within macromolecular complexes, the rules that drive segregation into collectives have yet to be defined. Similar kinds of phase transitions to those described here have recently been observed for other soluble proteins within the cytosol. For example, these kinds of transitions have been found for cohesive FG repeat domains in nuclear pore complexes, biogenesis of germline P granules, and in the assembly of other cytocortex signalling complexes.
To dismiss the complexity of myelination as mere conduction speed-up through insulation and capacitance decrease, is to ignore important clues about how the brain is organized, and how it progresses through structural changes. Neuroscientists generally give little pause to inspect some of the more basic geometric incidentals of myelination, like for example, the direction of myelin wrap. In proceeding down an axon, do successive myelin segments tend to wrap in the same direction? If they do, is it outlandish to suggest that one contributing factor to the presently-inexplicable process of myelin wrapping (which appears to progress at the innermost wrap), would be to posit that axons might exert a miniscule torque, perhaps cyctoskeletally mediated and membrane-attached as they fire?
Do all of the "arms" of a given oligodendrocyte prefer to wrap in the same orientation? Artistic liberty on this question has not yet been borne out by scientific study. When myelinating processes first extend, they are cytoskeletally-driven. At the cell body, we know that preferred polarities exist, perhaps centrosomally instructed, and that they are critical for organizing the structure of the cell. At what point if any, do these directional influences become isotropic, and by implication what power or biases over axon structure in the white matter might oligodendrocytes possess?
From an evolutionary viewpoint, much remains to be discovered about the origins of oligodendrocytes in the central nervous system, and the Schwann cells in the peripheral. Undoubtedly they share cell lineage origins, developmental programs, and protein toolkits, yet we still lack an understanding of the trade-offs involved in the single-cell myelination of Schwann cells vs the multi-armed approach of oligodendrocytes.
Often in biology, as has also been the case here, a single gene knockout study comes along and throws cold water on a painstakingly-constructed mechanism of action for a certain protein. Indeed MBP can not be the whole story for myelination because knockout mice can still myelinate, at least to a limited extent. The saving grace here is that, usually, redundant and complimentary mechanisms reveal themselves in the form of a different isoforms, or hither-to unknown genes that kick in to take up the slack in the mutant animal. Often these backups act in much the same way that the original pathway operated. Other times, as is seen in the myriad ways that developing embryos can coordinate critical events, completely different physics is employed.
The question of where the energy for myelination arises still remains. Entropic forces have been shown to play a role in many areas of biology, particularly certain kinds of phase changes, and may have utility here also. Much work on the temperature transitions and fluidity of myelin lipids has been done, as has work on exosomal transfer of components between axons and their myelinators. Combining work and ideas in these different areas will be of great value in understanding the larger story of myelin, and its function in the brain.
More information: PLoS Biol 11(6): e1001577. doi:10.1371/journal.pbio.1001577
© 2013 Phys.org