Epigenomic changes are key to innate immunological memory
A research team led by Keisuke Yoshida and Shunsuke Ishii of the RIKEN Molecular Genetics Laboratory has revealed that epigenomic changes induced by pathogen infections, mediated by a transcription factor called ATF7, are the underlying mechanism of the memory of innate immunity.
It was long believed that acquired immunity—a type of immunity mediated by T- and B-cells—had memory, meaning that it could learn from new pathogens, making subsequent reactions more effective, whereas innate immunity—which is mediated by macrophages and other types of cells that react to certain molecules typically associated with pathogens—did not. However, it gradually became clear that things were not so simple. Plants and insects, which only have innate immunity, also seem to have immunological memory. Further, it has been reported that herpes virus infection increases the resistance against bacteria in vertebrates. These phenomena suggest that innate immunity also has memory, but researchers have been reluctant to accept the hypothesis given the lack of a mechanism Now, in research published in Nature Immunology, a research team led by Keisuke Yoshida and Shunsuke Ishii of the RIKEN Molecular Genetics Laboratory has revealed that epigenomic changes induced by pathogen infections, mediated by a transcription factor called ATF7, are the underlying mechanism of the memory of innate immunity.
The research began from the discovery that in ATF7 knockout mice, macrophages appear similar to wild-type macrophages that have been activated by exposure to molecules that occur commonly in infections. The group had previously reported that ATF7-related transcription factors mediated epigenomic changes induced by heat shock or psychological stress, and that these changes were maintained for long periods after the exposure to the stress. Therefore, they speculated that infections by pathogens could induce epigenome changes in macrophages via ATF7.
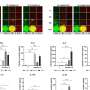
The group discovered that ATF7 binds to a group of innate immune genes and by doing so silences their expression, making the cell less responsive to infections. However, upon administration of lipopolysaccharidel (LPS), a molecule found in the outer membrane of Gram-negative bacteria, into mice, ATF7 was phosphorylated, weakening its activity so that immune-related genes were no longer silenced. Shunsuke Ishii, who led the group, says, "We were intrigued to find that even three weeks after the administration, the genes still showed increased activation. In mice, this status was shown to lead to increased resistance to Staphylococcus aureus, a Gram-positive bacteria."
According to Ishii, this finding could increase our understanding of what is known as the "hygiene hypothesis"—the concept that pathogen infection and unhygienic environment during infancy reduces the risk of allergy later in life. This hypothesis has been put forward to explain why the incidence of allergies and asthma is increasing around the world despite better hygienic conditions. "Though many researchers believe the hypothesis," says Ishii, "there is great uncertainty about how pathogen infection is memorized until adulthood. Since our research demonstrates that the pathogen-induced epigenomic changes mediated by ATF7 are maintained for a long period, this provides a plausible explanation of how the changes are induced. It also means that the genes that are affected can be used for the diagnosis of allergy."
Another possible application of these findings is for the choice of adjuvants in vaccines. Adjuvants—the name used for substances that activate innate immunity—are a necessary ingredient of efficient vaccines. The effect of adjuvant has generally been thought to end within a few days, but the present research showed that its effect can be maintained for longer periods. Says Ishii, "These results could affect the selection method of adjuvants, and we hope that they will contribute to the development of more efficient vaccines."
More information: The transcription factor ATF7 mediates lipopolysaccharide-induced epigenetic changes in macrophages involved in innate immune memory, DOI: 10.1038/ni.3257