How do we sense moonlight? Daylight? There's a cell for that
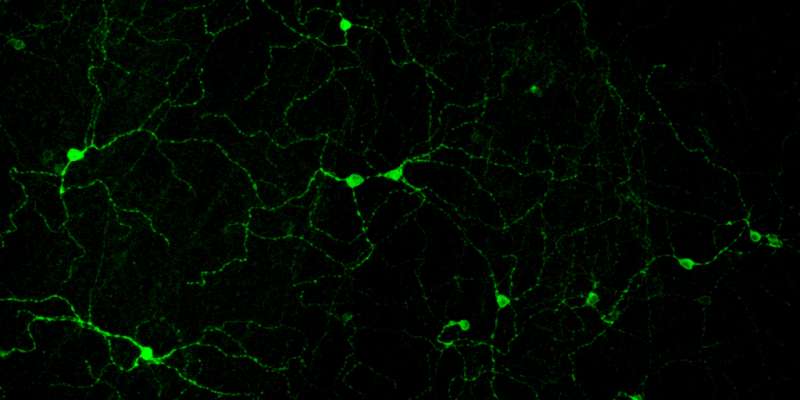
Reporting in today's Cell, neuroscientists at Boston Children's Hospital describe an unexpected way that we sense the overall degree of illumination in our environment. They found that neurons in the retina of the eye divvy up the job, with particular neurons tuned to different ranges of light intensity.
"As the earth turns, the level of illumination ranges across many orders of magnitude, from starlight to full daylight," says Michael Do, PhD, of the F.M. Kirby Neurobiology Center at Boston Children's Hospital, senior author on the paper. "How do you build a sensory system that covers such a broad range? It seems like a straightforward problem, but the solution we found was a lot more complex than expected."
Separate from the retina's rods and cones, which primarily detect form and movement, are other light-sensing neurons specialized for "non-image" vision—used to set our body clocks, regulate sleep and control hormone levels. These neurons, known as M1 ganglion cell photoreceptors, function even in people who are otherwise blind.
Elliott Milner, a PhD student in Harvard's Program in Neuroscience and lead author on the paper, established new methods to study the electrical outputs of these M1 cells. This allowed a better understanding of the signals these cells send from the eye to areas throughout the brain.
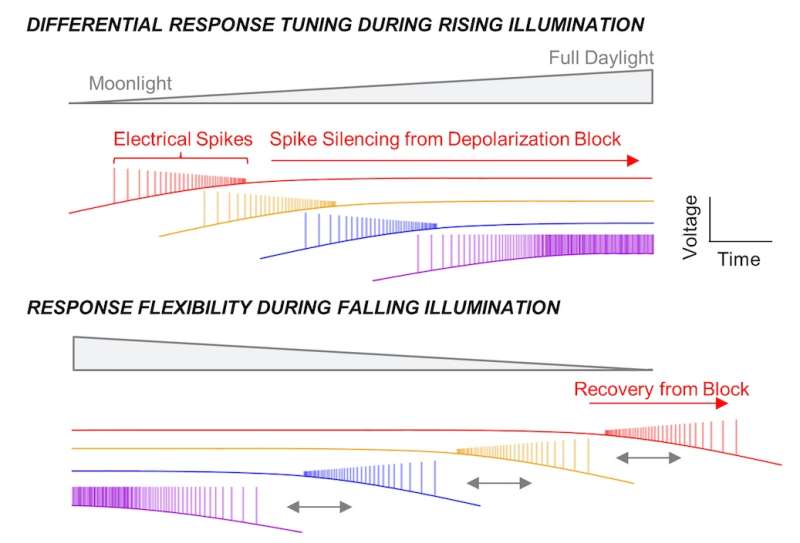
"The expectation from prior work was that signaling from these cells would simply increase with brightness, and that averaging across them would provide a measure of the overall light intensity," says Milner.
But what Milner and Do found was that although the cells appear visually indistinguishable from each other, they are tuned to respond to different light levels, and take turns signaling to the brain as these levels change. As a result, the brain gets information about light intensity from the identities of the cells that are active, not just signal size.
"Some cells signal vigorously in twilight and others in full daylight," says Milner. "Together, they cover a broad range of light intensities in the environment."
Leveraging a "pathological" phenomenon
Interestingly, the M1 cells' turn-taking system uses a mechanism that is usually considered abnormal or pathological, known as depolarization block.
As the light level goes up, a protein called melanopsin in the M1 cells captures more and more photons of light. This causes the voltage across the cell membrane to become more positive—that is, it "depolarizes." As the voltage becomes more positive, the cell generates more electrical spikes (also known as action potentials), which are the signals that are sent to the brain.
In depolarization block, typically observed in certain disorders like epilepsy, the cell loses its ability to fire spikes when the membrane voltage gets too positive. "There's so much excitation that the cell can't keep up and it goes silent," says Do.
The M1 cells seem to be using this feature to advantage. Milner and Do think this system may have evolved to help the brain distinguish light levels more precisely, based on which cells are "talking" and not only their general volume. It also may conserve energy.
"Spikes are expensive metabolically for a cell to produce," Do explains. "Because some cells are silenced as others activate, this system provides information at a lower energetic cost."
In future work, Milner and Do hope to explore the following questions:
- How does the brain extract information about the light level from these cells? Do particular regions of the brain listen to some M1 cells and not others?
- How are the different M1 cells distributed across the retina? Different parts of the retina receive light from different directions, for example, so how M1 cells are distributed spatially could have implications for designing light therapy systems for conditions like seasonal affective disorder.
- Do other cells involved in sensory perception—such as those that allow us to sense odors or touch—also use depolarization block?
"The bottom line is that nerve cells have more in their toolkit than we previously thought, and divide labor in ways we didn't expect," says Do. "We're finding surprises even in systems that are considered to be quite simple, like the one used to sense light intensity."