Scientists make new discoveries in the origin and ID of pancreatic endocrine cells
The pancreas is a key metabolic regulator. When pancreatic beta cells cease producing enough insulin, blood sugar levels rise dangerously—a phenomenon known as hyperglycemia—thus triggering diabetes. After discovering that other mature pancreatic cells can adapt and partly compensate for the lack of insulin, a team from the University of Geneva (UNIGE) demonstrates that the stem cells from which beta cells are derived are only present during embryonic development. This discovery puts an end to a long-standing controversy about the hypothetical existence of adult pancreatic stem cells that would give rise to newly differentiated hormone-producing cells after birth. The scientists also succeeded in precisely defining the 'identity card' of pancreatic endocrine cells, which is a promising tool for the production of replacement insulin-secreting cells. These results can be read in Cell Reports and Nature Communications.
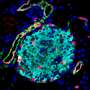
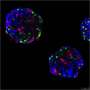
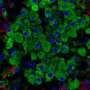
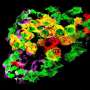
Diabetes is a common metabolic disease. It is characterized by a persistent hyperglycemia that occurs when pancreatic cells responsible for the production of insulin—the beta cells—are destroyed or are no longer able to produce this regulatory hormone in sufficient quantities. Since 2010, studies performed by the team of Pedro Herrera, a professor in the Department of Genetic Medicine and Development and in the Diabetes Centre at the UNIGE Faculty of Medicine, as well as at the Geneva Institute of Genetics and Genomics (iGE3), reveal that the other pancreatic endocrine cells—namely alpha, delta and gamma cells, which produce other hormones useful for the metabolic balance—can "learn" to produce insulin when beta cells are absent or defective. This phenomenon, observed in mice and humans, demonstrates the plasticity of pancreatic cells and paves the way to new therapeutic strategies.
The short life of pancreatic endocrine stem cells
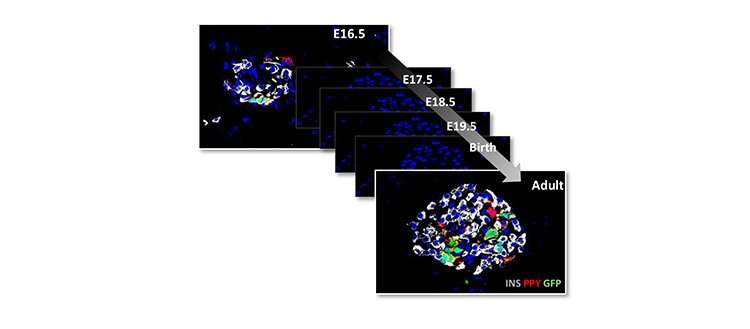
In two recent publications, Pedro Herrera's team reports new advances in the knowledge of the mechanisms of pancreatic cell formation, and in the gene expression profile defining the identity of each of the different islet endocrine cell types. The first research, published and featured in the cover of Cell Reports, demonstrates that pancreatic endocrine cells all derive from undifferentiated progenitor cells that emerge exclusively during embryonic development, but not after birth.
"Until now, some scientists thought that intra-pancreatic stem cells persist throughout life, "explains Pedro Herrera. Marta Perez-Frances, a researcher in Pedro Herrera's team and first author of this study, adds that their "work shows that this is not the case. Indeed, all pancreatic hormone-producing cells that emerge after birth originate from the division of existing differentiated cells that were generated during embryonic and fetal life from undifferentiated progenitor cells."
To decipher this mechanism, the scientists generated mouse models in which the different types of pancreatic endocrine cells were genetically tagged with a fluorescent tracer at different developmental stages in order to track them down after birth. The cells were traced up to the age of ten months, when mice are old.
A detailed ID card
In a second article, published in Nature Communications, Pedro Herrera's team focus on the gene expression profile of pancreatic endocrine cells. "Precisely defining the 'identity card' of these cells now helps us to design a tool aiming at engineering cell replacement therapies to treat diabetes. Such therapies could for instance consist of in vitro manufacturing of insulin-producing cells, or in stimulating pancreatic regeneration by exploiting the plasticity of the non-beta cells that we have discovered", explains Léon van Gurp, first author and post-doctoral fellow in the lab of Pedro Herrera.
The generation of surrogate cells with stable functional identities is crucial for the development of cell-based therapies to treat degenerative diseases. However, their generation requires reliable tools to accurately assess cell identities. To this aim, the researchers performed an extensive meta-analysis of single-cell transcriptomics, i.e. the analysis of the genes expressed by individual endocrine cells isolated from human pancreatic islets (these cells are grouped in small "clusters" within the pancreas). The identification of robustly expressed gene-sets allow the precise definition of the genetic signature of each endocrine cell type of the pancreas.
"Our work has no immediate clinical translation. Yet, by deciphering in detail the mechanisms governing the construction of cellular identities, it paves the way for developing innovative therapeutic approaches for treating diabetes and other pathologies linked to the loss of any given cell type," concludes Pedro Herrera.
The gene lists that the authors have generated can be downloaded from the Molecular Signatures Database.
More information: Marta Perez-Frances et al, Adult pancreatic islet endocrine cells emerge as fetal hormone-expressing cells, Cell Reports (2022). DOI: 10.1016/j.celrep.2022.110377
Léon van Gurp et al, Generation of human islet cell type-specific identity genesets, Nature Communications (2022). DOI: 10.1038/s41467-022-29588-8
Molecular Signatures Database: www.gsea-msigdb.org/gsea/msigdb