This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Human lung chip leveraged to faithfully model radiation-induced lung injury
The lung is one of the tissues most sensitive to radiation in the human body. People exposed to high radiation doses following nuclear incidents develop radiation-induced lung injury (RILI), which affects the function of many cell types in the lung, causing acute and sustained inflammation, and in the longer term, the thickening and scarring of lung tissue known as fibrosis.
RILI also is a common side effect of radiation therapy administered to cancer patients to kill malignant cells in their bodies, and can limit the maximum radiation dose doctors can use to control their tumors, as well as dramatically impair patients' quality of life.
Anti-inflammatory drugs given to patients during radiation therapy can dampen the inflammation in the lungs, called pneumonitis, but not all patients respond equally well. This is because RILI is a complex disorder that varies between patients and is influenced by risk factors such as age, lung cancer state, and other pre-existing lung diseases, and likely the patient's genetic makeup.
In the event of nuclear accidents, which usually involve the one-time exposure to much higher doses of radiation, no medical countermeasures are available yet that could prevent and protect against the damage to the lungs and other organs, making this a key priority of the US Food and Drug Administration (FDA).
A major obstacle to developing a much deeper understanding of the pathological processes triggered by radiation in the lung and other organs—which is the basis for discovering medical countermeasures—is the lack of experimental model systems that recapitulate how exactly the damage occurs in people.
Small animal preclinical models fail to produce key hallmarks of the human pathophysiology and do not mimic the dose sensitivities observed in humans. And although non-human primate models are considered the gold-standard for radiation injury, they are in short supply, costly, and raise serious ethical concerns; they also are not human and sometimes fail to predict responses observed when drugs move into the clinic.
Now, a multi-disciplinary research team at the Wyss Institute for Biologically Inspired Engineering at Harvard University and Boston Children's Hospital, led by Wyss Founding Director Donald Ingber, M.D., Ph.D., has developed a human in vitro model that closely mimics the complexities of RILI and radiation dose sensitivity of the human lung.
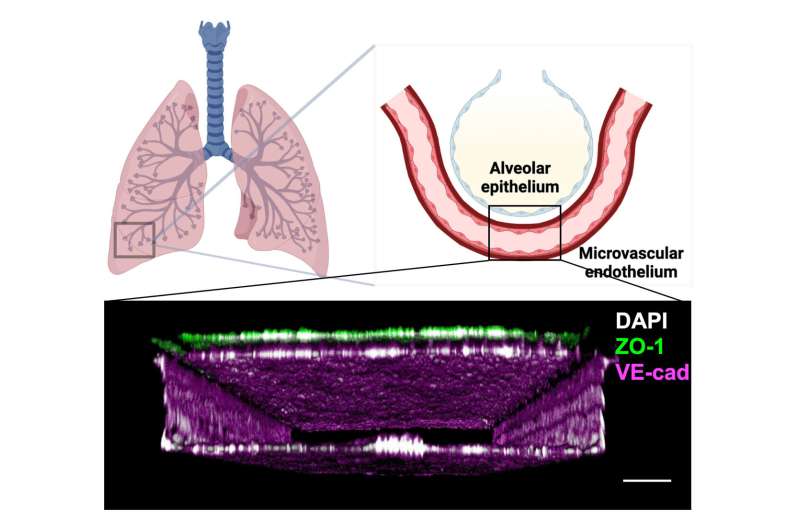
Lung alveoli are the small air sacs where oxygen and CO2 exchange between the lung and blood takes place, and the major site of radiation pneumonitis. Using a previously developed microfluidic human Lung Alveolus Chip lined by human lung alveolar epithelial cells interfaced with lung capillary cells to recreate the alveolar-capillary interface in vitro, the researchers recapitulated many of the hallmarks of RILI, including radiation-induced DNA damage in lung tissue, cell-specific changes in gene expression, inflammation, and injury to both the lung epithelial cells and blood vessel-lining endothelial cells.
By also evaluating the potential of two drugs to suppress the effects of acute RILI, the researchers demonstrated their model's capabilities as an advanced, human-relevant, preclinical drug discovery platform. The findings are published in Nature Communications.
"Forming a better understanding of how radiation injury occurs and finding new strategies to treat and prevent it poses a multifaceted challenge that in the face of nuclear threats and the realities of current cancer therapies needs entirely new solutions," said Ingber. "The Lung Chip model that we developed to recapitulate development of RILI leverages our extensive microfluidic organ chip culture expertise, and in combination with new analytical and computational drug and biomarker discovery tools, gives us powerful new inroads into this problem."
Ingber is also the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children's Hospital, and the Hansjörg Wyss Professor of Bioinspired Engineering at the Harvard John A. Paulson School of Engineering and Applied Sciences.
Advanced human in vitro model of RILI
The human Lung Alveolus Chip is a 2-channel microfluidic culture system in which primary human lung alveolar epithelial cells are cultured in one channel where they are exposed to air as they would be in the lung. They are also interfaced across a porous membrane with primary human lung capillary endothelial cells in the parallel channel that are constantly perfused with a blood-like nutrient medium that contains circulating human immune cells, which also can contribute to radiation responses.
This carefully engineered, immunologically active, alveolar-capillary interface also experiences cyclic mechanical movements mimicking actual breathing motions. Importantly, this living breathing Lung Chip can be transiently exposed to clinically relevant doses of radiation, and then investigated for the effects over an extended period of time.
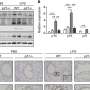
When the Lung Alveolus Chip was exposed to increasing doses of radiation, the cell, tissue, and organ-level responses modeled on-chip closely aligned with clinical observations, and importantly, offered new insights into RILI. Following a one-time radiation treatment, the team could observe breaks in the cells' chromosomes with activation of associated DNA repair machinery, whose numbers increased with the amount of radiation applied to the Lung Alveolus Chip.
This was paralleled by increased levels of reactive oxygen species that are known to also damage DNA, as well as many other types of molecules. Cells started to increase their size, a phenomenon known as hypertrophy that is commonly seen in alveolar injury in vivo; the barrier comprised by tightly packed endothelial and epithelial cells started to break down and liquid flowed through the endothelial channel accumulated in the epithelial channel.
"Interestingly, we mapped the levels of multiple pro-inflammatory cytokines over a seven-day course following the radiation treatment, which is especially important for assessing oncoming radiation injury to the lung. RILI symptoms in patients only begin to appear after a week following exposure, but the preceding inflammation cannot be captured in patients," said first-author Queeny Dasgupta, Ph.D., who led the project as a Postdoctoral Fellow on Ingber's team, and now is a Scientist at Systemic Bio, a 3D Systems company. "Once overt RILI has manifested itself in patients, it is often too late to rescue the affected lung tissue."
The team found that the first pro-inflammatory cytokines had already started to be upregulated six hours following the application of high radiation doses, and that their numbers and levels kept increasing until day seven, the end of their observation period.
Importantly, this trend was much stronger pronounced in vascular endothelial cells than lung epithelial cells. In parallel, the cellular injury in the Lung Alveolus Chip was reversed in epithelial cells over time, but in contrast, it was sustained in endothelial cells, replicating clinical observations that found RILI to predominantly impact the vascular endothelium in alveoli.
From radiation injury to genes to targets
To more systematically understand the cellular changes triggered by radiation in the Lung Chip, and identify potential drug targets, the researchers analyzed the complete gene expression programs of epithelial and endothelial cells over time. This allowed them to not only further define the cell-specific early and later-stage inflammatory responses, but also to generate whole-genome gene expression data that they could feed into a machine learning-based computational algorithm called "Network Model for Causality-Aware Discovery" (NeMoCAD).
NeMoCAD had previously enabled Ingber's team to predict therapeutic targets and repurpose drugs that reverse disease states. The analysis led them to home in on a gene called HMOX1, which is involved in an antioxidant response, and whose expression was upregulated immediately following radiation exposure and remained elevated throughout the seven-day course of the investigation.
"We found that further increasing HMOX1 levels with the drug lovastatin in the Lung Alveolus Chip reduced DNA damage and cellular hypertrophy early after the radiation, similarly to the anti-inflammatory drug prednisolone, which we used as a positive control. Later, however, lovastatin worsened the disruption of the endothelial barrier. In fact, by experimentally knocking down HMOX1 expression during later stages, we could partially reverse its later adverse effects," explained Dasgupta.
"This showed that HMOX1 function indeed is very relevant to the development of RILI, but also suggests that targeting HMOX1 and perhaps targets related to other potential processes might require a more balanced therapeutic approach."
The study is part of a larger campaign at the Wyss Institute aiming to investigate acute radiation damage across several organs and tissues. The group has previously modeled acute radiation injury in organ chip models of intestine and bone marrow as well, and each exhibited a different radiation dose sensitivity that matched that of humans.
Ingber's team also speculates that radiation damage in one organ might also affect the function of other organs and plans to address this possibility by microfluidically linking different organ chips in the future. In addition, they believe that the hypersensitivities of patients predisposed by other lung diseases to radiation could be modeled in personalized Lung Alveolus Chips.
Other authors on the study are past and present members of Ingber's team, including Amanda Jiang, Amy Wen, Robert Mannix, Yuncheng Man, Sean Hall, and Emilia Javorsky.
More information: Queeny Dasgupta et al, A human lung alveolus-on-a-chip model of acute radiation-induced lung injury, Nature Communications (2023). DOI: 10.1038/s41467-023-42171-z