Possible culprits in congenital heart defects identified
Mitochondria are the power plants of cells, manufacturing chemical fuel so a cell can perform its many tasks. These cellular power plants also are well known for their role in ridding the body of old or damaged cells.
Now, researchers at Washington University School of Medicine in St. Louis and the University of Padua-Dulbecco Telethon Institute in Italy have shown that mitochondria remarkably also orchestrate events that determine a cell's future, at least in the embryonic mouse heart. The new study identifies new potential genetic culprits in the origins of some congenital heart defects.
The study appears Oct. 3 in Science Express.
"This study is surprising because biologists always assumed that organ development was orchestrated from the nucleus of a cell, and that the mitochondria simply followed along and did what they were told," said Gerald W. Dorn II, MD, the Philip and Sima K. Needleman Professor of Medicine at Washington University. "Now we've shown that mitochondria can direct what type of tissue a cell will become during embryonic development. Until recently, I would have dismissed this notion as science fiction."
Indeed, Dorn compares the new findings to the science fiction film Invasion of the Body Snatchers. The current understanding of mitochondrial origins is that they began as independent bacteria that invaded other cells as parasites. This initial invasion of host cells evolved into a symbiotic relationship such that now cells rely on mitochondria for fuel and quality control and mitochondria rely on cells for their very existence.
"We knew our cells had developed a working relationship with what originally had been outside invaders," Dorn said. "But now we have evidence that these invaders can become the boss. Mitochondria are already known to be the gatekeepers of cell death. Our new study shows they also orchestrate cell destiny as heart muscle cells."
Though this study is specific to cardiac muscle cells, Dorn anticipates future work will investigate whether this paradigm is true in other cell types as well.
"These results reverse our understanding of cellular development," Dorn said. "It's not the nucleus controlling the mitochondria. In this instance, it's the mitochondria controlling nuclear gene expression and doing it in such a manner that it prevents cardiac muscle cells from developing."
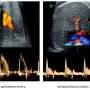
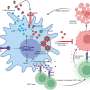
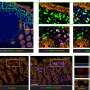
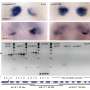
In normal development, mitochondria fuse together via proteins on their surface called Mitofusins 1 and 2 (Mfn1 and Mfn2). In this study, the researchers showed that deleting these two genes only in early heart muscle cells resulted in mouse embryos with severely underdeveloped hearts. The heart muscle walls were described as paper-thin. They further showed that mouse embryonic stem cells missing Mfn2 and Opa1, another gene with a similar role, could not develop into beating heart muscle cells.
Using a tissue culture technique he calls "hearts in the tube," Luca Scorrano, MD, PhD, professor of biochemistry at the University of Padua-Dulbecco Telethon Institute in Italy said, "We were able to unravel a novel way used by the ancient invader to take control of cell fate."
The researchers were careful to note that the underdeveloped hearts were not simply the result of a lack of fuel from dysfunctional mitochondria. Scorrano's team showed that these fragmented mitochondria, small and separated because they could not fuse together, interrupt well-known signaling pathways that govern how the nucleus expresses genes. Several of these signaling pathways already are implicated in congenital heart defects, such as those that result in holes in the wall separating the left and right ventricles, called ventricular septal defects.
"This study has opened up a new door that will permit us to expand our search for culprit genes in congenital heart defects, especially defects where the heart muscle is small and underdeveloped," Dorn said. "In particular, we need to be looking for mutations in the mitochondrial fusion proteins, including Mfn1 and 2."
In addition, Scorrano said, "It suggests that we should be looking at subtle heart problems in patients affected by Charcot-Marie-Tooth IIa and by Dominant Optic Atrophy, the two genetic diseases caused by mutations in Mfn2 and Opa1."
More information: Kasahara A, Cipolat S, Chen Y, Dorn GW, Scorrano L. Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin/notch signaling. Science Express. Oct. 3, 2013.